Putting Mechanically Interlocked Molecules (MIMs) to Work in Tomorrow’s World
The Golden Jubilee of the introduction by Gottfried Schill and Arthur Lüttringhaus in 1964 of the newest of (strong) bonds in chemistry at the time is being celebrated this year as the mechanical bond makes its transition from adolescence to adulthood. Molecules, of which the catenanes and rotaxanes are the archetypal examples, with mechanical bonds have emerged during their 50-year lifespan with ever-increasing frequency—after an induction period that lasted almost two decades—from being little more than intellectual curiosities to all but a small esoteric band of academic outliers and a few fancy-free researchers in the forward-looking industrial corporate laboratories of the day, to becoming one of the cornerstones of molecular nanotechnology.1

The mechanical link pervades our everyday lives in countless different enclaves of human endeavor, not only for its artistic and technological connotations, but also for its unique molecular properties that make it suitable for a gamut of potential applications in contemporary society. Amongst the most compelling of these properties is its capacity, at a sub-nanometer level, to orchestrate molecular movements—in a world that is governed by Brownian motion—in multistate molecular systems, integrated or otherwise, using external energy inputs from physical, chemical, electrochemical, and photochemical sources.
Prior to the appearance of the seminal communication (Angew. Chem. Int. Ed. 1964, 3, 546) on “The Preparation of Catena Compounds by Directed Synthesis” by Schill and Lüttringhaus, chemists had put their faith by and large in statistical protocols for the production of catenanes and rotaxanes, only to find out over and over again that they were on a hiding to nothing. The key crystalline compound 11
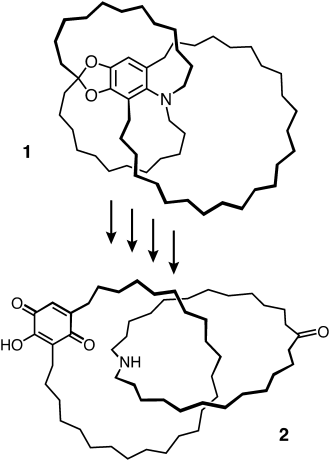
Gottfried Schill continued independently to develop this ground-breaking covalent directed synthesis methodology of mechanically interlocked molecules (MIMs) for many years to come, making the first wholly synthetic [3]catenanes and several [2]rotaxanes, while coming within an ace of crafting the first molecular trefoil knot and presciently coining the term translational isomerism in anticipation of what was in store stereochemically for MIMs. Schill’s celebrated monograph on Catenanes, Rotaxanes, and Knots published by Academic Press in 1971, together with his independent research in the two decades that followed, established him, in my mind at least, as the father of the mechanical bond. With hindsight, and reflecting on a 30-year expression of his creativity from 1961 to 1990, Gottfried Schill is a chemist who was decades ahead of his time!
While Schill’s directed synthesis, which relies on covalent templation, put the mechanical bond on a firm experimental footing, it has one major deficiency—namely, the lack of any significant intramolecular interactions between the components of the MIMs, following cleavage of the covalent template. Fortunately, a solution to this dilemma was at hand. At the same time as the mechanical bond was experiencing its growing pains, chemists were also witnessing a sea-change on the back of Charles Pedersen’s accidental discovery of the crown ethers in the 1960s. Jean-Marie Lehn and Donald Cram wasted no time at all in demonstrating the fundamental significance of molecular recognition and self-assembly processes under the auspices of what soon thereafter became known as supramolecular (host–guest) chemistry, which laid the foundations (Figure 1) for coordinative and noncovalent directed synthesis around (active) templates to produce highly programmed MIMs. As the timeline in Figure 2 illustrates, chemists of different persuasions have lost no time in devising protocols for the production of MIMs based on reactions conducted under both kinetic and thermodynamic control and guided by recognition elements that have explored almost every conceivable genre of intermolecular interaction as a source of templation, leading to the formation of MIMs with a wide variety of architectures containing single and multiple mechanical bonds. Suffice it to say that progress during the past quarter of a century has been fast and furious, partly because MIMs offer the unique opportunity to have many of the phenomena that characterize the supramolecular repertoire expressed within the comfort zone of a molecular setting.
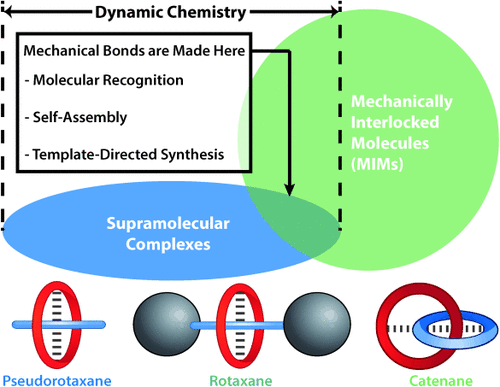
Supramolecular complexes provide the stepping-stone between a traditional and a contemporary molecular world. The hatched lines in the pseudorotaxane, rotaxane, and catenane indicate weak interactions.
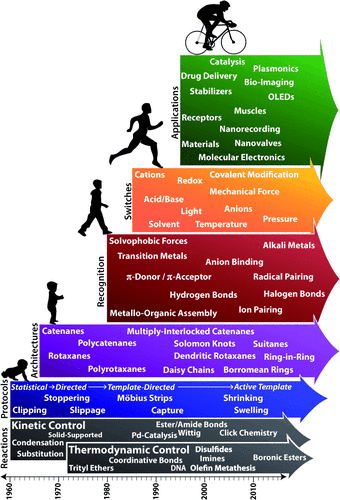
Timeline for the growth of research on the mechanical bond.
An attractive feature of MIMs from many vantage points is that they can readily be rendered bistable and, by playing one recognition site off against another in a bistable catenane or rotaxane, they become switchable at the behest of stimuli that can be electrochemical, as well as chemical and photochemical. Now that switches based on bistable MIMs, which have been designed and operated in solution and on surfaces during these past two decades, are a dime a dozen, the time has come for chemists—in cahoots with materials scientists and engineers—employing the mechanical bond in their research endeavors to focus more of their fundamental knowledge and practical expertise into seeking out applications for MIMs. Based on our extensive knowledge of the switches and machines that power devices and motors in today’s world, it is not beyond our ken and wits to envisage molecular electronic devices (MEDs) and artificial molecular machines (AMMs) running on electrochemical energy in tomorrow’s world. Nothing is stopping us putting mechanically interlocked molecules to work. Let life begin at 50 for MIMs! What better way of honoring pioneer Gottfried Schill on the occasion of the Golden Jubilee of the mechanical bond?





