Isolation of an Imino-N-heterocyclic Carbene/Germanium(0) Adduct: A Mesoionic Germylene Equivalent†
Bochao Su
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Nanyang Link 21,Singapore 637371 (Singapore)
Search for more papers by this authorDr. Rakesh Ganguly
NTU-CBC Crystallography Facility, Nanyang Technological University (Singapore)
Search for more papers by this authorDr. Yongxin Li
NTU-CBC Crystallography Facility, Nanyang Technological University (Singapore)
Search for more papers by this authorCorresponding Author
Prof. Dr. Rei Kinjo
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Nanyang Link 21,Singapore 637371 (Singapore)
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Nanyang Link 21,Singapore 637371 (Singapore)Search for more papers by this authorBochao Su
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Nanyang Link 21,Singapore 637371 (Singapore)
Search for more papers by this authorDr. Rakesh Ganguly
NTU-CBC Crystallography Facility, Nanyang Technological University (Singapore)
Search for more papers by this authorDr. Yongxin Li
NTU-CBC Crystallography Facility, Nanyang Technological University (Singapore)
Search for more papers by this authorCorresponding Author
Prof. Dr. Rei Kinjo
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Nanyang Link 21,Singapore 637371 (Singapore)
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Nanyang Link 21,Singapore 637371 (Singapore)Search for more papers by this authorThe authors gratefully acknowledge financial support from Nanyang Technological University and PSF/A*STAR (SERC 1321202066) of Singapore.
Graphical Abstract
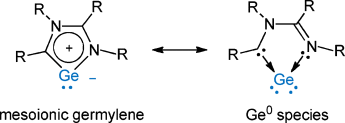
Germanium zero: Reduction of a chlorogermyliumylidene chelated by an imino-N-heterocyclic carbene ligand with potassium graphite produced a novel cyclic germenium species, which can be viewed as both a germanium(0) species and a mesoionic germylene. X-ray diffraction analysis and computational studies revealed one of the lone pairs on the Ge atom is involved in the π system on the GeC2N2 five-membered ring.
Abstract
An autoionization of germanium dichloride/dioxane complex with an imino-N-heterocyclic carbene ligand (L) afforded a novel germyliumylidene ion, [(L)GeCl]+[GeCl3]−, which was fully characterized. Reduction of the germyliumylidene ion with potassium graphite produced a cyclic species [(L)Ge], which can be viewed as both a Ge0 species and a mesoionic germylene. X-ray diffraction analysis and computational studies revealed one of the lone pairs on the Ge atom is involved in the π system on the GeC2N2 five-membered ring. It was also confirmed that the nucleophilic behavior of [(L)Ge] as a two lone-pair donor.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201406930_sm_miscellaneous_information.pdf401.9 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. Igau, H. Grutzmacher, A. Baceiredo, G. Bertrand, J. Am. Chem. Soc. 1988, 110, 6463–6466;
- 1bA. J. Arduengo III, R. L. Harlow, M. Kline, J. Am. Chem. Soc. 1991, 113, 361–363.
- 2For recent reviews, see:
- 2aM. N. Hopkinson, C. Richter, M. Schedler, F. Glorius, Nature 2014, 510, 485–496;
- 2bD. J. Nelson, S. P. Nolan, Chem. Soc. Rev. 2013, 42, 6723–6753;
- 2cT. Dröge, F. Glorius, Angew. Chem. Int. Ed. 2010, 49, 6940–6952; Angew. Chem. 2010, 122, 7094–7107;
- 2dA. J. Arduengo, G. Bertrand, Chem. Rev. 2009, 109, 3209–3210;
- 2eF. E. Hahn, M. C. Jahnke, Angew. Chem. Int. Ed. 2008, 47, 3122–3172; Angew. Chem. 2008, 120, 3166–3216.
- 3
- 3aD. Martin, M. Melaimi, M. Soleilhavoup, G. Bertrand, Organometallics 2011, 30, 5304–5313;
- 3bM. Melaimi, M. Soleihavoup, G. Bertrand, Angew. Chem. Int. Ed. 2010, 49, 8810–8849; Angew. Chem. 2010, 122, 8992–9032.
- 4E. Aldeco-Perez, A. J. Rosenthal, B. Donnadieu, P. Parames-waran, G. Frenking, G. Bertrand, Science 2009, 326, 556–559.
- 5R. H. Crabtree, Coord. Chem. Rev. 2013, 257, 755–766; see also Ref. [2c] and [3].
- 6For recent reviews, see:
- 6aM. Asay, C. Jones, M. Driess, Chem. Rev. 2011, 111, 354–396;
- 6bY. Mizuhata, T. Sasamori, N. Tokitoh, Chem. Rev. 2009, 109, 3479–3511;
- 6cM. F. Lappert, P. P. Power, A. Protchenko, A. Seeber, Metal Amide Chemistry, Wiley, Chichester, 2009;
- 6dA. V. Zabula, F. E. Hahn, Eur. J. Inorg. Chem. 2008, 5165–5179.
- 7For NHC-stabilized diatomic group 14 element allotropes, see:
- 7aC. Jones, A. Sidiropoulos, N. Holzmann, G. Frenking, A. Stasch, Chem. Commun. 2012, 48, 9855–9857;
- 7bA. Sidiropoulos, C. Jones, A. Stasch, S. Klein, G. Frenking, Angew. Chem. Int. Ed. 2009, 48, 9701–9704; Angew. Chem. 2009, 121, 9881–9884;
- 7cY. Wang, Y. Xie, P. Wei, R. B. King, H. F. Schäfer III, P. V. R. Schleyer, G. H. Robinson, Science 2008, 321, 1069–1071;
- 7dC. A. Dyker, G. Bertrand, Science 2008, 321, 1050–1051.
- 8N. Wiberg, H.-W. Lerner, S.-K. Vasisht, S. Wagner, K. Karaghiosoff, H. Nöth, W. Ponikwar, Eur. J. Inorg. Chem. 1999, 1211–1218.
10.1002/(SICI)1099-0682(199908)1999:8<1211::AID-EJIC1211>3.0.CO;2-0 CAS Web of Science® Google Scholar
- 9
- 9aM. Kira, Chem. Commun. 2010, 46, 2893–2903;
- 9bM. Kira, T. Iwamoto, S. Ishida, H. Masuda, T. Abe, C. Kabuto, J. Am. Chem. Soc. 2009, 131, 17135–17144;
- 9cT. Iwamoto, H. Masuda, C. Kabuto, M. Kira, Organometallics 2005, 24, 197–199;
- 9dS. Ishida, T. Iwamoto, C. Kabuto, M. Kira, Nature 2003, 421, 725–727; For another example of trisilaallene without structural characterization, see:
- 9eH. Tanaka, S. Inoue, M. Ichinohe, M. Driess, A. Sekiguchi, Organometallics 2011, 30, 3475–3478.
- 10
- 10aK. C. Mondal, H. W. Roesky, M. C. Schwarzer, G. Frenking, B. Niepotter, H. Wolf, R. Herbst-Irmer, D. Stalke, Angew. Chem. Int. Ed. 2013, 52, 2963–2967; Angew. Chem. 2013, 125, 3036–3040;
- 10bY. Li, K. C. Mondal, H. W. Roesky, H. Zhu, P. Stollberg, R. Herbst-Irmer, D. Stalke, D. M. Andrada, J. Am. Chem. Soc. 2013, 135, 12422–12428.
- 11
- 11aY. Xiong, S. Yao, G. Tan, S. Inoue, M. Driess, J. Am. Chem. Soc. 2013, 135, 5004–5007;
- 11bY. Xiong, S. Yao, S. Inoue, J. D. Epping, M. Driess, Angew. Chem. Int. Ed. 2013, 52, 7147–7150; Angew. Chem. 2013, 125, 7287–7290.
- 12
- 12aT. Chu, L. Belding, A. van der Est, T. Dudding, I. Korobkov, G. I. Nikonov, Angew. Chem. Int. Ed. 2014, 53, 2711–2715; Angew. Chem. 2014, 126, 2749–2753; For a Sn analogue, see:
- 12bJ. Flock, A. Suljanovic, A. Torvisco, W. Schoefberger, B. Gerke, R. Pöttgen, R. C. Fischer, M. Flock, Chem. Eur. J. 2013, 19, 15504–15517.
- 13
- 13aP. Liu, M. Wesolek, A. A. Danopoulos, P. Braunstein, Organometallics 2013, 32, 6286–6297;
- 13bJ. A. Thagfi, G. G. Lavoie, Organometallics 2012, 31, 7351–7358;
- 13cT. G. Larocque, A. C. Badaj, S. Dastgir, G. G. Lavoie, Dalton Trans. 2011, 40, 12705–12712;
- 13dJ. A. Thagfi, S. Dastgir, A. J. Lough, G. G. Lavoie, Organometallics 2010, 29, 3133–3138.
- 14CCDC 1012364 (2), 1012365 (3), 1012366 (4), and 1018493 (5) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 15A. P. Singh, H. W. Roesky, E. Carl, D. Stalke, J.-P. Demers, A. Lange, J. Am. Chem. Soc. 2012, 134, 4998–5003.
- 16
- 16aS. Khan, G. Gopakumar, W. Thiel, M. Alcarazo, Angew. Chem. Int. Ed. 2013, 52, 5644–5647; Angew. Chem. 2013, 125, 5755–5758;
- 16bY. Xiong, S. Yao, S. Inoue, A. Berkefeld, M. Driess, Chem. Commun. 2012, 48, 12198–12200;
- 16cF. Cheng, J. D. Dyke, F. Ferrante, A. L. Hector, W. Levason, G. Reid, M. Webster, W. Zhang, Dalton Trans. 2010, 39, 847–856;
- 16dT. Probst, O. Steigelmann, J. Riede, H. Schmidbaur, Angew. Chem. Int. Ed. Engl. 1990, 29, 1397–1398; Angew. Chem. 1990, 102, 1471–1473.
- 17Gaussian 09, M. J. Frisch et al., Gaussian, Inc., Wallingford, CT, 2010 (for full reference, see the Supporting Information).
- 18For different viewpoints on the use of dative arrows, see:
- 18aD. Himmel, I. Krossing, A. Schnepf, Angew. Chem. Int. Ed. 2014, 53, 370–374; Angew. Chem. 2014, 126, 378–382;
- 18bG. Frenking, Angew. Chem. Int. Ed. 2014, 53, 6040–6046; Angew. Chem. 2014, 126, 6152–6158;
- 18cD. Himmel, I. Krossing, A. Schnepf, Angew. Chem. Int. Ed. 2014, 53, 6047–6049; Angew. Chem. 2014, 126, 6159–6160.
- 19
- 19aR. West, H. Sohn, D. R. Powell, T. Müller, Y. Apeloig, Angew. Chem. Int. Ed. 1996, 35, 1002–1004; Angew. Chem. 1996, 108, 1095–1097;
- 19bW. P. Freeman, T. D. Tilley, L. M. Liable-Sand, A. L. Rheingold, J. Am. Chem. Soc. 1996, 118, 10457–10468; For review, see:
- 19cV. Ya. Lee, A. Sekiguchi, Angew. Chem. Int. Ed. 2007, 46, 6596–6620; Angew. Chem. 2007, 119, 6716–6740.
- 20
- 20aV. Ya. Lee, A. Sekiguchi, Comprehensive Inorganic Chemistry II, Vol.1, Elsevier, Amsterdam, 2013, pp. 289–324;
10.1016/B978-0-08-097774-4.00113-3 Google Scholar
- 20bR. C. Fischer, P. P. Power, Chem. Rev. 2010, 110, 3877–3923;
- 20cV. Ya. Lee, A. Sekiguchi, Organometallic Compounds of Low-Coordinate Si, Ge, Sn and Pb: From Phantom Species to Stable Compounds, Wiley, Chichester, 2010, Chapter 5.
10.1002/9780470669266 Google Scholar
- 21
- 21aW. A. Herrmann, M. Denk, J. Behm, W. Scherer, F.-R. Klingan, H. Bock, B. Solouki, M. Wagner, Angew. Chem. Int. Ed. Engl. 1992, 31, 1485–1488; Angew. Chem. 1992, 104, 1489–1492; For reviews, see Ref. [6].