Diversity-Oriented Synthesis of Drug-Like Macrocyclic Scaffolds Using an Orthogonal Organo- and Metal Catalysis Strategy†
Dr. André Grossmann
Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge CB2 1EW (UK) http://www-spring.ch.cam.ac.uk/
Search for more papers by this authorSean Bartlett
Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge CB2 1EW (UK) http://www-spring.ch.cam.ac.uk/
Search for more papers by this authorMatej Janecek
Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge CB2 1EW (UK) http://www-spring.ch.cam.ac.uk/
Search for more papers by this authorDr. James T. Hodgkinson
Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge CB2 1EW (UK) http://www-spring.ch.cam.ac.uk/
Search for more papers by this authorCorresponding Author
Prof. David R. Spring
Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge CB2 1EW (UK) http://www-spring.ch.cam.ac.uk/
Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge CB2 1EW (UK) http://www-spring.ch.cam.ac.uk/Search for more papers by this authorDr. André Grossmann
Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge CB2 1EW (UK) http://www-spring.ch.cam.ac.uk/
Search for more papers by this authorSean Bartlett
Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge CB2 1EW (UK) http://www-spring.ch.cam.ac.uk/
Search for more papers by this authorMatej Janecek
Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge CB2 1EW (UK) http://www-spring.ch.cam.ac.uk/
Search for more papers by this authorDr. James T. Hodgkinson
Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge CB2 1EW (UK) http://www-spring.ch.cam.ac.uk/
Search for more papers by this authorCorresponding Author
Prof. David R. Spring
Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge CB2 1EW (UK) http://www-spring.ch.cam.ac.uk/
Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge CB2 1EW (UK) http://www-spring.ch.cam.ac.uk/Search for more papers by this authorOur work was supported by the European Union, Engineering and Physical Sciences Research Council, Biotechnology and Biological Sciences Research Council, Medical Research Council, Cancer Research UK, and the Wellcome Trust. A.G. thanks the German Research Foundation for a postdoctoral fellowship (GR 4429/1-1). S.B. thanks the Herchel Smith Fund for a Ph.D. studentship.
Graphical Abstract
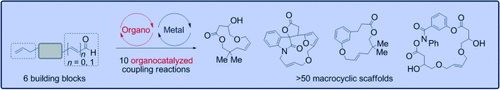
Hand in hand: The outlined diversity-oriented synthesis of a library of macrocycles is based on the orthogonal combination of multiple diversity-generating organocatalytic steps with alkene metathesis. In total, 51 macrocyclic structures bearing 48 unique scaffolds, drug-like chemophysical properties, and natural-product-like shape diversity were synthesized in only 2 to 4 steps without the need for protecting groups.
Abstract
Small-molecule modulators of biological targets play a crucial role in biology and medicine. In this context, diversity-oriented synthesis (DOS) provides strategies toward generating small molecules with a broad range of unique scaffolds, and hence three-dimensionality, to target a broad area of biological space. In this study, an organocatalysis-derived DOS library of macrocycles was synthesized by exploiting the pluripotency of aldehydes. The orthogonal combination of multiple diversity-generating organocatalytic steps with alkene metathesis enabled the synthesis of 51 distinct macrocyclic structures bearing 48 unique scaffolds in only two to four steps without the need for protecting groups. Furthermore, merging organocatalysis and alkene metathesis in a one-pot protocol facilitated the synthesis of drug-like macrocycles with natural-product-like levels of shape diversity in a single step.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201406865_sm_miscellaneous_information.pdf19.4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1M. Kaiser, S. Wetzel, K. Kumar, H. Waldmann, Cell. Mol. Life Sci. 2008, 65, 1186–1201.
- 2C. J. O’Connor, L. Laraia, D. R. Spring, Chem. Soc. Rev. 2011, 40, 4332–4345.
- 3M. Kawasumi, P. Nghiem, J. Invest. Dermatol. 2007, 127, 1577–1584.
- 4D. A. Spiegel, F. C. Schroeder, J. R. Duvall, S. L. Schreiber, J. Am. Chem. Soc. 2006, 128, 14766–14767.
- 5D. P. Walsh, Y. T. Chang, Chem. Rev. 2006, 106, 2476–2530.
- 6W. R. J. D. Galloway, A. Bender, M. Welch, D. R. Spring, Chem. Commun. 2009, 2446–2462.
- 7L. Ruddigkeit, R. van Deursen, L. C. Blum, J.-L. Reymond, J. Chem. Inf. Model. 2012, 52, 2864–2875.
- 8W. R. J. D. Galloway, D. R. Spring, Expert Opin. Drug Discovery 2009, 4, 467–472.
- 9W. R. J. D. Galloway, A. Isidro-Llobet, D. R. Spring, Nat. Commun. 2010, 1, 80.
- 10S. J. Haggarty, Curr. Opin. Chem. Biol. 2005, 9, 296–303.
- 11M. K. Schwarz, W. H. B. Sauer, J. Chem. Inf. Comput. Sci. 2003, 43, 987–1003.
- 12F. Lovering, J. Bikker, C. Humblet, J. Med. Chem. 2009, 52, 6752–6756.
- 13P. A. Clemons, N. E. Bodycombe, H. A. Carrinski, J. A. Wilson, A. F. Shamji, B. K. Wagner, A. N. Koehler, S. L. Schreiber, Proc. Natl. Acad. Sci. USA 2011, 107, 18787–18792.
- 14T. Luker et al., Bioorg. Med. Chem. Lett. 2011, 21, 5673–5679.
- 15T. J. Ritchie, S. J. F. Macdonald, Drug Discovery Today 2009, 14, 1011–1020.
- 16S. L. Schreiber, Science 2000, 287, 1964–1969.
- 17S. Oh, S. B. A. Park, Chem. Commun. 2011, 47, 12754–12761.
- 18G. L. Thomas et al., Angew. Chem. Int. Ed. 2008, 47, 2808–2812; Angew. Chem. 2008, 120, 2850–2854.
- 19B. Z. Stanton et al., Nat. Chem. Biol. 2009, 5, 154–156.
- 20R. W. Heidebrecht et al., ACS Med. Chem. Lett. 2012, 3, 112–117.
- 21S. Wetzel, R. S. Bon, K. Kumar, H. Waldmann, Angew. Chem. Int. Ed. 2011, 50, 10800–10826; Angew. Chem. 2011, 123, 10990–11018.
- 22A. P. Antonchick, C. Gerding-Reimers, M. Catarinella, M. Schürmann, H. Preut, S. Ziegler, D. Rauh, H. Waldmann, Nat. Chem. 2010, 2, 735–740.
- 23H. Dückert et al., Nat. Chem. Biol. 2011, 8, 179–184.
- 24B. M. Ibbeson, L. Laraia, E. Alza, C. J. O’Connor, Y. S. Tan, H. M. L. Davies, G. McKenzie, A. R. Venkitaraman, D. R. Spring, Nat. Commun. 2014, 5, 3155.
- 25T. E. Nielsen, S. L. Schreiber, Angew. Chem. Int. Ed. 2008, 47, 48–56; Angew. Chem. 2008, 120, 52–61.
- 26C. J. White, A. K. Yudin, Nat. Chem. 2011, 3, 509–524.
- 27B. Yoo, S. B. Y. Shin, M. L. Huang, K. Kirshenbaum, Chem. Eur. J. 2010, 16, 5528–5537.
- 28L. A. Marcaurelle, et al., J. Am. Chem. Soc. 2010, 132, 16962–16976.
- 29A. Isidro-Llobet, T. Murillo, P. Bello, A. Cilibrizzi, J. T. Hodgkinson, W. R. J. D. Galloway, A. Bender, M. Welch, D. R. Spring, Proc. Natl. Acad. Sci. USA 2011, 108, 6793–6798.
- 30R. A. Bauer, T. A. Wenderski, D. S. Tan, Nat. Chem. Biol. 2013, 9, 21–29.
- 31F. Kopp, C. F. Stratton, L. B. Akella, D. S. Tan, Nat. Chem. Biol. 2012, 8, 358–365.
- 32E. Comer, H. Liu, A. Joliton, A. Clabaut, C. Johnson, L. B. Akella, L. A. Marcaurelle, Proc. Natl. Acad. Sci. USA 2011, 108, 6751–6756.
- 33K. M. G. O’Connell, H. S. G. Beckmann, L. Laraia, H. T. Horsley, A. Bender, A. R. Venkitaraman, D. R. Spring, Org. Biomol. Chem. 2012, 10, 7545–7551.
- 34H. S. G. Beckmann, F. Nie, C. E. Hagerman, H. Johansson, Y. S. Tan, D. Wilcke, D. R. Spring, Nat. Chem. 2013, 5, 861–867.
- 35
- 35aE. M. Driggers, S. P. Hale, J. Lee, N. K. Terrett, Nat. Rev. Drug Discovery 2008, 7, 608–624;
- 35bJ. Mallinson, I. Collins, Future Med. Chem. 2012, 4, 1409–1438.
- 36Special Issue on Organocatalysis (Ed.: B. List): Chem. Rev. 2007, 107, 5413–5883.
- 37
- 37aD. Enders, O. Niemeier, A. Henseler, Chem. Rev. 2007, 107, 5606–5655;
- 37bV. Nair, R. S. Menon, A. T. Biju, C. R. Sinu, R. R. Paul, A. Jose, V. Sreekumar, Chem. Soc. Rev. 2011, 40, 5336–5346;
- 37cA. T. Biju, N. Kuhl, F. Glorius, Acc. Chem. Res. 2011, 44, 1182–1195;
- 37dH. U. Vora, P. Wheeler, T. Rovis, Adv. Synth. Catal. 2012, 354, 1617–1639.
- 38For selected examples on NHC-catalysed benzoin reaction of two equal aldehydes, see:
- 38aT. Ukai, S. Tanaka, S. Dokawa, J. Pharm. Soc. Jpn. 1943, 63, 296–300;
- 38bR. Breslow, J. Am. Chem. Soc. 1958, 80, 3719–3726;
- 38cD. Enders, U. Kallfass, Angew. Chem. Int. Ed. 2002, 41, 1743–1745;
10.1002/1521-3773(20020517)41:10<1743::AID-ANIE1743>3.0.CO;2-Q CAS PubMed Web of Science® Google ScholarAngew. Chem. 2002, 114, 1822–1824;
- 38dY. Ma, S. Wei, J. Wu, F. Yang, B. Liu, J. Lan, S. Yang, J. You, Adv. Synth. Catal. 2008, 350, 2645–2651;
- 38eL. Baragwanath, C. A. Rose, K. Zeitler, S. J. Connon, J. Org. Chem. 2009, 74, 9214–9217.
- 39For selected examples on NHC-catalysed Stetter reaction, see:
- 39aH. Stetter, M. Schrecke, Angew. Chem. Int. Ed. Engl. 1973, 12, 81; Angew. Chem. 1973, 85, 89;
- 39bM. Christmann, Angew. Chem. Int. Ed. 2005, 44, 2632–2634; Angew. Chem. 2005, 117, 2688–2690;
- 39cQ. Liu, S. Perreault, T. Rovis, J. Am. Chem. Soc. 2008, 130, 14066–14067;
- 39dD. Enders, J. Han, A. Henseler, Chem. Commun. 2008, 3989–3991;
- 39eQ. Liu, T. Rovis, Org. Lett. 2009, 11, 2856–2859;
- 39fD. A. DiRocco, K. M. Oberg, D. M. Dalton, T. Rovis, J. Am. Chem. Soc. 2009, 131, 10872–10874;
- 39gD. A. DiRocco, T. Rovis, J. Am. Chem. Soc. 2011, 133, 10402–10405;
- 39hT. Jousseaume, N. E. Wurz, F. Glorius, Angew. Chem. Int. Ed. 2011, 50, 1410–1414; Angew. Chem. 2011, 123, 1446–1450;
- 39iX. Fang, X. Chen, H. Lv, Y. R. Chi, Angew. Chem. Int. Ed. 2011, 50, 11782–11785; Angew. Chem. 2011, 123, 11986–11989;
- 39jD. A. DiRocco, E. L. Noey, K. N. Houk, T. Rovis, Angew. Chem. Int. Ed. 2012, 51, 2391–2394; Angew. Chem. 2012, 124, 2441–2444.
- 40
- 40aN. T. Reynolds, J. Read deAlaniz, T. Rovis, J. Am. Chem. Soc. 2004, 126, 9518–9519;
- 40bA. Chan, K. A. Scheidt, Org. Lett. 2005, 7, 905–908;
- 40cS. S. Sohn, J. W. Bode, Org. Lett. 2005, 7, 3873–3876;
- 40dN. T. Reynolds, T. Rovis, J. Am. Chem. Soc. 2005, 127, 16406–16407;
- 40eS. S. Sohn, J. W. Bode, Angew. Chem. Int. Ed. 2006, 45, 6021–6024; Angew. Chem. 2006, 118, 6167–6170;
- 40fJ. Kaeobamrung, J. Mahatthananchai, P. Zheng, J. W. Bode, J. Am. Chem. Soc. 2010, 132, 8810–8812;
- 40gA. D. Smith, K. B. Ling, Chem. Commun. 2011, 47, 373–375;
- 40hD. Enders, A. Grossmann, D. Van Craen, Org. Biomol. Chem. 2013, 11, 138–141.
- 41aV. Nair, S. Vellalath, M. Poonoth, R. Mohan, E. Suresh, Org. Lett. 2006, 8, 507–509;
- 41bH. Lv, B. Tiwari, J. Mo, C. Xing, Y. R. Chi, Org. Lett. 2012, 14, 5412–5415.
- 42aC. Burstein, F. Glorius, Angew. Chem. Int. Ed. 2004, 43, 6205–6208; Angew. Chem. 2004, 116, 6331–6334;
- 42bS. S. Sohn, E. L. Rosen, J. W. Bode, J. Am. Chem. Soc. 2004, 126, 14370–14371;
- 42cD. T. Cohen, C. C. Eichman, E. M. Phillips, E. R. Zarefsky, K. A. Scheidt, Angew. Chem. Int. Ed. 2012, 51, 7309–7313; Angew. Chem. 2012, 124, 7421–7425.
- 43aV. Nair, S. Vellalath, M. Poonoth, E. Suresh, J. Am. Chem. Soc. 2006, 128, 8736–8737;
- 43bP. Verma, P. A. Patni, R. B. Sunoj, J. Org. Chem. 2011, 76, 5606–5613;
- 43cM. Wadamoto, E. M. Phillips, T. E. Reynolds, K. A. Scheidt, J. Am. Chem. Soc. 2007, 129, 10098–10099.
- 44
- 44aV. Nair, C. R. Sinu, B. P. Babu, V. Varghese, A. Jose, E. Suresh, Org. Lett. 2009, 11, 5570–5573;
- 44bB. Maji, L. Ji, S. Wang, S. Vedachalam, R. Ganguly, X.-W. Liu, Angew. Chem. Int. Ed. 2012, 51, 8276–8280; Angew. Chem. 2012, 124, 8401–8405.
- 45For selected examples on NHC-catalysed cascade processes, see:
- 45aS. P. Lathrop, T. Rovis, J. Am. Chem. Soc. 2009, 131, 13628–13630;
- 45bB. Cardinal-David, D. E. A. Raup, K. A. Scheidt, J. Am. Chem. Soc. 2010, 132, 5345–5347;
- 45cD. E. A. Raup, B. Cardinal-David, D. Holte, K. A. Scheidt, Nat. Chem. 2010, 2, 766–771;
- 45dC. M. Filloux, S. P. Lathrop, T. Rovis, Proc. Natl. Acad. Sci. USA 2010, 107, 20666–20671;
- 45eN. T. Patil, Angew. Chem. Int. Ed. 2011, 50, 1759–1761; Angew. Chem. 2011, 123, 1797–1799;
- 45fD. T. Cohen, B. Cardinal-David, K. A. Scheidt, Angew. Chem. Int. Ed. 2011, 50, 1678–1682; Angew. Chem. 2011, 123, 1716–1720;
- 45gX. Song, Q. Ni, A. Grossmann, D. Enders, Chem. Asian J. 2013, 8, 2965–2969.
- 46For review on NHC-catalysed cascade processes, see: A. Grossmann, D. Enders, Angew. Chem. Int. Ed. 2012, 51, 314–325; Angew. Chem. 2012, 124, 320–332.
- 47An overview of all macrocyclic structures in the presented DOS library is given in the supporting information (pS3-pS6).
- 48Ł. Albrecht, H. Jiang, K. A. Jørgensen, Angew. Chem. Int. Ed. 2011, 50, 8492–8509; Angew. Chem. 2011, 123, 8642–8660.
- 49D. T. Cohen, K. A. Scheidt, Chem. Sci. 2012, 3, 53–57.
- 50K. Namitharan, T. Zhu, J. Cheng, P. Zheng, X. Li, S. Yang, B.-A. Song, Y. R. Chi, Nat. Commun. 2014, 5, 3982.
- 51X.-X. Liu, E. Pilarinou, J. L. McLaughlin, Tetrahedron Lett. 1999, 40, 399–402.
- 52In a preliminary phenotypic screen, compound 50 indicated activity against several bacterial strains. Results will be reported in due course.