Catalytic Asymmetric Functionalization of Aromatic CH Bonds by Electrophilic Trapping of Metal-Carbene-Induced Zwitterionic Intermediates†
Shikun Jia
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development; Department of Chemistry, East China Normal University, 3663, North Zhongshan Road, Shanghai, 200062 (China)
Search for more papers by this authorCorresponding Author
Dr. Dong Xing
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development; Department of Chemistry, East China Normal University, 3663, North Zhongshan Road, Shanghai, 200062 (China)
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development; Department of Chemistry, East China Normal University, 3663, North Zhongshan Road, Shanghai, 200062 (China)Search for more papers by this authorDan Zhang
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development; Department of Chemistry, East China Normal University, 3663, North Zhongshan Road, Shanghai, 200062 (China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Wenhao Hu
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development; Department of Chemistry, East China Normal University, 3663, North Zhongshan Road, Shanghai, 200062 (China)
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development; Department of Chemistry, East China Normal University, 3663, North Zhongshan Road, Shanghai, 200062 (China)Search for more papers by this authorShikun Jia
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development; Department of Chemistry, East China Normal University, 3663, North Zhongshan Road, Shanghai, 200062 (China)
Search for more papers by this authorCorresponding Author
Dr. Dong Xing
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development; Department of Chemistry, East China Normal University, 3663, North Zhongshan Road, Shanghai, 200062 (China)
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development; Department of Chemistry, East China Normal University, 3663, North Zhongshan Road, Shanghai, 200062 (China)Search for more papers by this authorDan Zhang
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development; Department of Chemistry, East China Normal University, 3663, North Zhongshan Road, Shanghai, 200062 (China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Wenhao Hu
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development; Department of Chemistry, East China Normal University, 3663, North Zhongshan Road, Shanghai, 200062 (China)
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development; Department of Chemistry, East China Normal University, 3663, North Zhongshan Road, Shanghai, 200062 (China)Search for more papers by this authorWe are grateful for financial support from NSF of China (20932003 and 21125209), the MOST of China (2011CB808600) and STCSM (12JC1403800).
Graphical Abstract
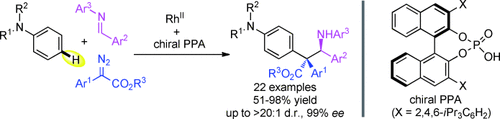
Caught in a trap: The title reaction of N,N-disubstituted anilines with diazo compounds and imines is reported for the efficient construction of α,α-diaryl benzylic quaternary stereocenters in good yields with high diastereoselectivities and excellent enantioselectivities. Efficient electrophilic trapping of the metal-carbene-induced zwitterionic intermediate is crucial for the enantiocontrol under RhII/chiral phosphoric acid (PPA) co-catalysis.
Abstract
Asymmetric functionalization of aromatic CH bonds of N,N-disubstituted anilines with diazo compounds and imines is reported for the efficient construction of α,α-diaryl benzylic quaternary stereocenters in good yields with high diastereoselectivities and excellent enantioselectivities. This RhII/chiral phosphoric acid cocatalyzed transformation is proposed to proceed through a metal-carbene-induced zwitterionic intermediate which undergoes electrophilic trapping. To the best of our knowledge, this is the first asymmetric example of metal carbene-induced intermolecular functionalization of aryl CH bonds.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201406492_sm_miscellaneous_information.pdf3.7 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For recent reviews on metal-carbene-induced CH functionalizations, see:
- 1aM. P. Doyle, M. A. McKervey, T. Ye, Modern Catalytic Methods for Organic Synthesis with Diazo Compounds, Wiley, New York, 1998;
- 1bH. M. L. Davies, R. E. J. Beckwith, Chem. Rev. 2003, 103, 2861;
- 1cH. M. L. Davies, J. R. Manning, Nature 2008, 451, 417;
- 1dM. P. Doyle, R. Duffy, M. Ratnikov, L. Zhou, Chem. Rev. 2010, 110, 704;
- 1eH. M. L. Davies, D. Morton, Chem. Soc. Rev. 2011, 40, 1857;
- 1fM. M. Díaz-Requejo, P. J. Pérez, Chem. Rev. 2008, 108, 3379;
- 1gC. N. Slattery, A. Ford, A. R. Maguire, Tetrahedron 2010, 66, 6681;
- 1hJ. Yamaguchi, A. D. Yamaguchi, K. Itami, Angew. Chem. Int. Ed. 2012, 51, 8960; Angew. Chem. 2012, 124, 9092;
- 1iC. Zheng, S.-L. You, RSC Adv. 2014, 4, 6173.
- 2For aromatic CH functionalization catalyzed by rhodium, see:
- 2aM. J. Rosenfeld, B. K. R. Shankar, H. Shechter, J. Org. Chem. 1988, 53, 2699;
- 2bM. Yang, T. R. Webb, P. Livant, J. Org. Chem. 2001, 66, 4945.
- 3For aromatic CH functionalization catalyzed by copper, see:
- 3aE. Tayama, T. Yanaki, H. Iwamoto, E. Hasegawa, Eur. J. Org. Chem. 2010, 6719–6721;
- 3bE. Tayama, M. Ishikawa, H. Iwamoto, E. Hasegawa, Tetrahedron Lett. 2012, 53, 5159.
- 4For aromatic CH functionalization catalyzed by gold, see:
- 4aM. R. Fructos, T. R. Belderrain, P. de Frémont, N. M. Scott, S. P. Nolan, M. M. Díaz-Requejo, P. J. Pérez, Angew. Chem. Int. Ed. 2005, 44, 5284; Angew. Chem. 2005, 117, 5418;
- 4bI. Rivilla, B. P. Gómez-Emeterio, M. R. Fructos, M. M. Díaz-Requejo, P. J. Pérez, Organometallics 2011, 30, 2855;
- 4cZ. Yu, B. Ma, M. Chen, H.-H. Wu, L. Liu, J. Zhang, J. Am. Chem. Soc. 2014, 136, 6904;
- 4dY. Xi, Y. Su, Z. Yu, B. Dong, E. J. McClain, Y. Lan, X. Shi, Angew. Chem. Int. Ed. 2014, DOI: ; Angew. Chem. 2014, DOI: .
- 5The functionalization of aromatic CH bonds with diazo compounds by a rhodium(III)-catalyzed chelation-assisted CH activation pathway has recently been realized. See:
- 5aW.-W. Chan, S.-F. Lo, Z. Zhou, W.-Y. Yu, J. Am. Chem. Soc. 2012, 134, 13565;
- 5bT. K. Hyster, K. E. Ruhl, T. Rovis, J. Am. Chem. Soc. 2013, 135, 5364;
- 5cZ. Shi, D. C. Koester, M. Boultadakis-Arapinis, F. Glorius, J. Am. Chem. Soc. 2013, 135, 12204;
- 5dS. Cui, Y. Zhang, D. Wang, Q. Wu, Chem. Sci. 2013, 4, 3912;
- 5eF. Hu, Y. Xia, F. Ye, Z. Liu, C. Ma, Y. Zhang, J. Wang, Angew. Chem. Int. Ed. 2014, 53, 1364; Angew. Chem. 2014, 126, 1388.
- 6
- 6aN. Watanabe, Y. Ohtake, S.-i. Hashimoto, M. Shiro, S. Ikegami, Tetrahedron Lett. 1995, 36, 1491;
- 6bN. Watanabe, T. Ogawa, Y. Ohtake, S. Ikegami, S.-i. Hashimoto, Synlett 1996, 85;
- 6cC. A. Merlic, A. L. Zechman, M. M. Miller, J. Am. Chem. Soc. 2001, 123, 11101;
- 6dH. Tsutsui, Y. Yamaguchi, S. Kitagaki, S. Nakamura, M. Anada, S. Hashimoto, Tetrahedron: Asymmetry 2003, 14, 817.
- 7
- 7aA. DeAngelis, V. W. Shurtleff, O. Dmitrenko, J. M. Fox, J. Am. Chem. Soc. 2011, 133, 1650;
- 7bY. Cai, S. F. Zhu, G. P. Wang, Q. L. Zhou, Adv. Synth. Catal. 2011, 353, 2939;
- 7cH. Qiu, D. Zhang, S. Liu, L. Qiu, J. Zhou, Y. Qian, C. Zhai, W. Hu, Acta Chim. Sin. 2012, 70, 2484;
- 7dY. Lian, H. M. L. Davies, Org. Lett. 2012, 14, 1934;
- 7eY. Lian, H. M. L. Davies, J. Am. Chem. Soc. 2010, 132, 440.
- 8
- 8aH. M. L. Davies, E. G. Antoulinakis, J. Organomet. Chem. 2001, 617–618, 45;
- 8bH. M. L. Davies, J. Mol. Catal. A 2002, 189, 125.
- 9For an excellent example to demonstrate the difference between chiral rhodium complex catalyzed C(sp3)H and C(sp2)H functionalizations, see: H. M. L. Davies, Q. Jin, Org. Lett. 2004, 6, 1769.
- 10For selected leading reviews on enantioselective protonation, see:
- 10aJ. T. Mohr, A. Y. Hong, B. M. Stoltz, Nat. Chem. 2009, 1, 359;
- 10bC. Fehr, Angew. Chem. Int. Ed. Engl. 1996, 35, 2566; Angew. Chem. 1996, 108, 2726;
- 10cL. Duhamel, P. Duhamel, J.-C. Plaquevent, Tetrahedron: Asymmetry 2004, 15, 3653.
- 11For reviews and selected examples developed by our group, see:
- 11aX. Guo, W. Hu, Acc. Chem. Res. 2013, 46, 2427;
- 11bD. Xing, W. Hu, Tetrahedron Lett. 2014, 55, 777;
- 11cW. H. Hu, X. F. Xu, J. Zhou, W. J. Liu, H. X. Huang, J. Hu, L. P. Yang, L. Z. Gong, J. Am. Chem. Soc. 2008, 130, 7782;
- 11dJ. Jiang, H.-D. Xu, J.-B. Xi, B.-Y. Ren, F.-P. Lv, X. Guo, L.-Q. Jiang, Z.-Y. Zhang, W.-H. Hu, J. Am. Chem. Soc. 2011, 133, 8428;
- 11eH. Qiu, M. Li, L.-Q. Jiang, F.-P. Lv, L. Zan, C.-W. Zhai, M. P. Doyle, W.-H. Hu, Nat. Chem. 2012, 4, 733.
- 12For recent examples developed by others, see:
- 12aB. Alcaide, P. Almendros, C. Aragoncillo, R. Callejo, M. P. Ruiz, M. R. Torres, J. Org. Chem. 2009, 74, 8421;
- 12bC.-Y. Zhou, J.-C. Wang, J. Wei, Z.-J. Xu, Z. Guo, K.-H. Low, C.-M. Che, Angew. Chem. Int. Ed. 2012, 51, 11376; Angew. Chem. 2012, 124, 11538;
- 12cB. Alcaide, P. Almendros, C. Aragoncillo, R. Callejo, M. P. Ruiz, M. R. Torres, Eur. J. Org. Chem. 2012, 2359;
- 12dL. Ren, X.-L. Lian, L.-Z. Gong, Chem. Eur. J. 2013, 19, 3315;
- 12eL.-Y. Mei, X.-Y. Tang, M. Shi, Org. Biomol. Chem. 2014, 12, 1149.
- 13For reviews and selected examples of chiral phosphoric acid catalyzed asymmetric addition reaction to imines, see:
- 13aT. Akiyama, Chem. Rev. 2007, 107, 5744;
- 13bA. G. Doyle, E. N. Jacobsen, Chem. Rev. 2007, 107, 5713;
- 13cJ. M. M. Verkade, L. J. C. v. Hemert, P. J. L. M. Quaedflieg, F. P. J. T. Rutjes, Chem. Soc. Rev. 2008, 37, 29;
- 13dD. Uraguchi, M. Terada, J. Am. Chem. Soc. 2004, 126, 5356;
- 13eT. Akiyama, J. Itoh, K. Yokota, K. Fuchibe, Angew. Chem. Int. Ed. 2004, 43, 1566; Angew. Chem. 2004, 116, 1592.
- 14For selected reviews of the combination of transition-metal catalysis and organocatalysis, see:
- 14aZ. Shao, H. Zhang, Chem. Soc. Rev. 2009, 38, 2745;
- 14bC. Zhong, X. Shi, Eur. J. Org. Chem. 2010, 2999;
- 14cZ. Du, Z. Shao, Chem. Soc. Rev. 2013, 42, 1337.
- 15aJ. W. C. Skokie, H. W. S. Deerfield, Patent 3225054, 1965;
- 15bR. A. Stokbroekx, J. Vandenberk, A. H. M. T. Van Heertum, G. M. L. W. Van Laar, M. J. M. C. Van der Aa, W. F. M. Van Bever, P. A. J. Janssen, J. Med. Chem. 1973, 16, 782;
- 15cE. M. Schultz, C. M. Robb, J. M. Sprague, J. Am. Chem. Soc. 1947, 69, 2454;
- 15dM. C. Lu, W. E. Wung, L. B. Shih, S. Callejas, J. E. Gearien, E. B. Thompson, J. Med. Chem. 1987, 30, 273.
- 16We choose substituted anilines or phenols as the arene sources for the reason that the heterosubstitution at the para-position may be beneficial to the generation of the proposed zwitterionic intermediates. For example, see Ref. [9].
- 17For details, see the Supporting Information.
- 18S. Caddick, N. J. Parr, M. C. Pritchard, Tetrahedron 2001, 57, 6615.
- 19L. Simón, J. M. Goodman, J. Org. Chem. 2011, 76, 1775.
Citing Literature
November 24, 2014
Pages 13098-13101