Monitoring Endocytic Trafficking of Anthrax Lethal Factor by Precise and Quantitative Protein Labeling†
We thank Prof. Feng Shao (NIBS, China) for donation of plasmids and Dr. Rong Meng (Peking University, China) for MS detection. This work was supported by the National Basic Research Program of China (2010CB912302 and 2012CB917301) and the National Natural Science Foundation of China (21225206 and 91313301).
Graphical Abstract
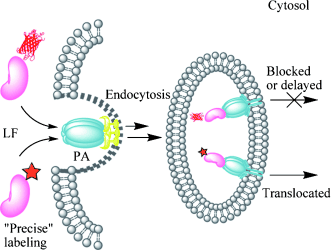
Visualizing toxin trafficking: Coupling the genetic-code expansion strategy with bioorthogonal reactions allowed site-specific fluorescent labeling of anthrax lethal factor (LF) with little perturbation to its native structure and function. Time-lapse visualization of the endocytic trafficking of a precisely labeled LF revealed molecular details underlying its virulence mechanism inside host cells. PA=protective antigen.
Abstract
Coupling the genetic code expansion technique with bioorthogonal reactions enables precise control over the conjugation site as well as the choice of fluorescent probes during protein labeling. However, the advantages of this strategy over bulky and rigid fluorescent proteins (FPs) remain to be fully explored. Here we applied site-specific bioorthogonal labeling on anthrax lethal factor (LF) to visualize its membrane translocation inside live cells. In contrast to the previously reported FP tags that significantly perturbed LF’s membrane trafficking, our precisely and quantitatively labeled LF exhibited an endocytic activity comparable to wild-type LF. This allowed time-lapse imaging of LF’s natural translocation process from host cell membrane to cytosol, which revealed molecular details of its virulence mechanism. Our strategy is generally applicable for monitoring intracellular protein membrane translocation that is difficult to access using conventional protein labeling methodologies.