Monobenzofused 1,4-Azaborines: Synthesis, Characterization, and Discovery of a Unique Coordination Mode†
Dr. Senmiao Xu
Department of Chemistry, Boston College, Chestnut Hill, MA 02467 (USA)
Search for more papers by this authorDr. Fredrik Haeffner
Department of Chemistry, Boston College, Chestnut Hill, MA 02467 (USA)
Search for more papers by this authorDr. Bo Li
Department of Chemistry, Boston College, Chestnut Hill, MA 02467 (USA)
Search for more papers by this authorDr. Lev N. Zakharov
Center for Advanced Materials Characterization in Oregon, University of Oregon, Eugene, OR 97403-1253 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Shih-Yuan Liu
Department of Chemistry, Boston College, Chestnut Hill, MA 02467 (USA)
Department of Chemistry, Boston College, Chestnut Hill, MA 02467 (USA)Search for more papers by this authorDr. Senmiao Xu
Department of Chemistry, Boston College, Chestnut Hill, MA 02467 (USA)
Search for more papers by this authorDr. Fredrik Haeffner
Department of Chemistry, Boston College, Chestnut Hill, MA 02467 (USA)
Search for more papers by this authorDr. Bo Li
Department of Chemistry, Boston College, Chestnut Hill, MA 02467 (USA)
Search for more papers by this authorDr. Lev N. Zakharov
Center for Advanced Materials Characterization in Oregon, University of Oregon, Eugene, OR 97403-1253 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Shih-Yuan Liu
Department of Chemistry, Boston College, Chestnut Hill, MA 02467 (USA)
Department of Chemistry, Boston College, Chestnut Hill, MA 02467 (USA)Search for more papers by this authorCorrespondence concerning X-ray crystallography should be directed to Lev N. Zakharov ([email protected]) and Bo Li (complex 8 h, 11; [email protected]). Correspondence concerning electronic structure calculations should be directed to Fredrik Haeffner ([email protected]). Portions of this work were carried out at the University of Oregon. Support for this work has been provided by the National Science Foundation (CHE-1212346) and by Universal Display Corporation.
Graphical Abstract
Abstract
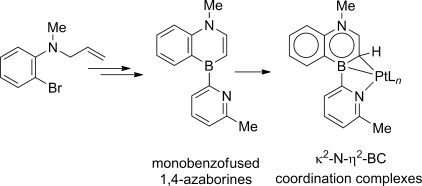
We report the first general synthesis of boron-substituted monobenzofused 1,4-azaborines using ring-closing metathesis of an enamine-containing diene as a key synthetic strategy. As part of our investigations, we discovered that the B-C3 moiety in a 1,4-azaborine can serve uniquely as a η2-L-type ligand. This functionality is exemplified by two κ2-N-η2-BC Pt complexes of a boron-pyridyl-substituted monobenzofused-1,4-azaborine. Single-crystal X-ray diffraction analysis of the Pt complexes shows a strong structural contribution from the iminium resonance form of the monobenzofused-1,4-azaborine ligand. We also demonstrate that a palladium(0) complex supported by a 1,4-azaborine-based phosphine ligand can catalyze hydroboration of 1-buten-3-yne with unique selectivity. In view of the importance of arene–metal π-interactions in catalytic applications, this work should open new opportunities for ligand design involving the 1,4-azaborine motif as an arene substitute.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201403903_sm_miscellaneous_information.pdf6.3 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For an overview, see:
- 1aZ. Liu, T. B. Marder, Angew. Chem. 2008, 120, 248–250; Angew. Chem. Int. Ed. 2008, 47, 242–244;
- 1bM. J. D. Bosdet, W. E. Piers, Can. J. Chem. 2009, 87, 8–29;
- 1cP. G. Campbell, A. J. V. Marwitz, S.-Y. Liu, Angew. Chem. 2012, 124, 6178–6197;
10.1002/ange.201200063 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 6074–6092.
- 2For pioneering work, see:
- 2aM. J. S. Dewar, V. P. Kubba, R. Pettit, J. Chem. Soc. 1958, 3073–3076;
- 2bM. J. S. Dewar, R. Dietz, J. Chem. Soc. 1959, 2728–2730;
- 2cM. J. S. Dewar, P. A. Marr, J. Am. Chem. Soc. 1962, 84, 3782.
- 3For recent work, see:
- 3aA. J. Ashe III, X. Fang, Org. Lett. 2000, 2, 2089–2091;
- 3bA. J. Ashe III, X. Fang, X. Fang, J. W. Kampf, Organometallics 2001, 20, 5413–5418;
- 3cA. N. Lamm, S.-Y. Liu, Mol. BioSyst. 2009, 5, 1303–1305;
- 3dA. J. V. Marwitz, S. P. McClintock, L. N. Zakharov, S.-Y. Liu, Chem. Commun. 2010, 46, 779–781;
- 3eT. Taniguchi, S. Yamaguchi, Organometallics 2010, 29, 5732–5735;
- 3fA. N. Lamm, E. B. Garner, D. A. Dixon, S.-Y. Liu, Angew. Chem. 2011, 123, 8307–8310;
10.1002/ange.201103192 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 8157–8160;
- 3gT. Hatakeyama, S. Hashimoto, S. Seki, M. Nakamura, J. Am. Chem. Soc. 2011, 133, 18614–18617;
- 3hA. J. V. Marwitz, A. N. Lamm, L. N. Zakharov, M. Vasiliu, D. A. Dixon, S. Y. Liu, Chem. Sci. 2012, 3, 825–829;
- 3iD. H. Knack, J. L. Marshall, G. P. Harlow, A. Dudzik, M. Szaleniec, S. Y. Liu, J. Heider, Angew. Chem. 2013, 125, 2660–2662;
10.1002/ange.201208351 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 2599–2601;
- 3jJ. S. Lu, S. B. Ko, N. R. Walters, Y. Kang, F. Sauriol, S. Wang, Angew. Chem. 2013, 125, 4642–4646; Angew. Chem. Int. Ed. 2013, 52, 4544–4548;
- 3kG. E. Rudebusch, L. N. Zakharov, S.-Y. Liu, Angew. Chem. 2013, 125, 9486–9489;
10.1002/ange.201304443 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 9316–9319.
- 4
- 4aS. Xu, L. N. Zakharov, S.-Y. Liu, J. Am. Chem. Soc. 2011, 133, 20152–20155;
- 4bS. Xu, T. C. Mikulas, L. N. Zakharov, D. A. Dixon, S.-Y. Liu, Angew. Chem. 2013, 125, 7675–7679; Angew. Chem. Int. Ed. 2013, 52, 7527–7531.
- 51,2-Azaborine, 1,3-azaborine, and 1,4-azaborine are abbreviations for 1,2-dihydro-1,2-azaborine, 1,3-dihydro-1,3-azaborine, and 1,4-dihydro-1,4-azaborine, respectively.
- 6
- 6aM. Kranz, F. Hampel, T. Clark, J. Chem. Soc. Chem. Commun. 1992, 1247–1248;
- 6bT. Agou, M. Sekine, J. Kobayashi, T. Kawashima, Chem. Commun. 2009, 1894–1896;
- 6cT. Agou, H. Arai, T. Kawashima, Chem. Lett. 2010, 39, 612–613.
- 7H. Braunschweig, A. Damme, J. O. Jimenez-Halla, B. Pfaffinger, K. Radacki, J. Wolf, Angew. Chem. 2012, 124, 10177–10180; Angew. Chem. Int. Ed. 2012, 51, 10034–10037.
- 8
- 8aA. J. V. Marwitz, E. R. Abbey, J. T. Jenkins, L. N. Zakharov, S.-Y. Liu, Org. Lett. 2007, 9, 4905–4908;
- 8bE. R. Abbey, A. N. Lamm, A. W. Baggett, L. N. Zakharov, S.-Y. Liu, J. Am. Chem. Soc. 2013, 135, 12908–12913.
- 9S. Krompiec, M. Pigulla, M. Krompiec, B. Marciniec, D. Chadyniak, J. Mol. Catal. A 2005, 237, 17–25.
- 10The presence of an electron-rich N-vinyl group could be problematic for the RCM reaction because of possible formation of a stable Fisher-type carbene complex, see: Z. Wu, S. T. Nguyen, R. H. Grubbs, J. W. Ziller, J. Am. Chem. Soc. 1995, 117, 5503–5511.
- 11For examples of ring-closing metathesis reactions of enamide-type substrates, see:
- 11aS. S. Kinderman, J. H. van Maarseveen, H. E. Schoemaker, H. Hiemstra, F. P. J. T. Rutjes, Org. Lett. 2001, 3, 2045–2048;
- 11bM. L. Bennasar, T. Roca, M. Monerris, D. García-Díaz, J. Org. Chem. 2006, 71, 7028–7034;
- 11cM. Arisawa, Y. Terada, K. Takahashi, M. Nakagawa, A. Nishida, J. Org. Chem. 2006, 71, 4255–4261.
- 12Full conversion was observed by 11B NMR spectroscopy.
- 13See Supporting Information for details.
- 14Monomer 8 f was not observed at elevated temperature whereas an appreciable amount of [(8 g)2] was observed at low temperature. The equilibrium constant was obtained by variable temperature (VT) 1H NMR spectroscopy. See Figure S2–S4, Table S1, and Chart S1 in the Supporting Information for details.
- 15Note, the treatment of 9 with HCl did not lead to the corresponding protonated monomer.
- 16Z. M. Hudson, B. A. Blight, S. Wang, Org. Lett. 2012, 14, 1700–1703.
- 17Complex 11 is thermally unstable. Upon heating 11 to 50 °C, metallic Pt0 could be observed, consistent with some form of reductive elimination reactivity. C3H activation by Pt was not observed.
- 18For key references, see:
- 18aF. R. Hartley, Chem. Rev. 1969, 69, 799–844;
- 18bN. Weding, M. Hapke, Chem. Soc. Rev. 2011, 40, 4525–4538;
- 18cW. D. Harman, Chem. Rev. 1997, 97, 1953–1978.
- 19For example, Pd0–arene interactions have been proposed to improve the catalytic activity in cross-coupling reactions, see:
- 19aJ. Yin, M. P. Rainka, X. X. Zhang, S. L. Buchwald, J. Am. Chem. Soc. 2002, 124, 1162–1163;
- 19bS. M. Reid, R. C. Boyle, J. T. Mague, M. J. Fink, J. Am. Chem. Soc. 2003, 125, 7816–7817;
- 19cS. D. Walker, T. E. Barder, J. R. Martinelli, S. L. Buchwald, Angew. Chem. 2004, 116, 1907–1912; Angew. Chem. Int. Ed. 2004, 43, 1871–1876;
- 19dT. E. Barder, S. D. Walker, J. R. Martinelli, S. L. Buchwald, J. Am. Chem. Soc. 2005, 127, 4685–4696.
- 20For Reviews, see:
- 20aH. Braunschweig, R. D. Dewhurst, A. Schneider, Chem. Rev. 2010, 110, 3924–3957;
- 20bD. J. Emslie, B. E. Cowie, K. B. Kolpin, Dalton Trans. 2012, 41, 1101–1117.
- 21For pioneering work, see:
- 21aM. M. Olmstead, P. P. Power, K. J. Weese, R. J. Doedens, J. Am. Chem. Soc. 1987, 109, 2541–2542;
- 21bK. S. Cook, W. E. Piers, S. J. Rettig, Organometallics 1999, 18, 1575–1577;
- 21cK. S. Cook, W. E. Piers, T. K. Woo, R. McDonald, Organometallics 2001, 20, 3927–3937;
- 21dK. S. Cook, W. E. Piers, R. McDonald, J. Am. Chem. Soc. 2002, 124, 5411–5418.
- 221H NMR spectrum of the reaction mixture indicates no reaction occurred between the carbonaceous naphthylpyridine ligand 12 and [{Pt(Me)2(μ-SMe2)}2]. See Figure S5 in Supporting Information for details.
- 23The carbonaceous naphthylpyridine ligand 12 and dibenzofused 1,4-azaborines do not furnish the corresponding η2-Pt complexes. See Supporting Information for details (Figure S6–S8).
- 24Crystallographic data for 9, [(8 g)2], 8 h, 10, 11, 14, 15, 16 a and 16 b can be found in the Supporting Information. CCDC 977735 (9), 977734 [(8 g)2], 990259 (8 h), 979025 (10), 977460 (11), 977857 (14), 978486 (15), 986967 (S16 a), and 986966 (S16 b) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 25The cationic Pt complex 15 is considered the more electron-deficient complex.
- 26A. Bondi, J. Phys. Chem. 1964, 68, 441–451.
- 27M. Mantina, A. C. Chamberlin, R. Valero, C. J. Cramer, D. G. Truhlar, J. Phys. Chem. A 2009, 113, 5806–5812.
- 28
- 28aM. Sircoglou, S. Bontemps, G. Bouhadir, N. Saffon, K. Miqueu, W. Gu, M. Mercy, C. H. Chen, B. M. Foxman, L. Maron, O. V. Ozerov, D. Bourissou, J. Am. Chem. Soc. 2008, 130, 16729–16738;
- 28bH. Braunschweig, A. Damme, T. Kupfer, Angew. Chem. 2011, 123, 7317–7320; Angew. Chem. Int. Ed. 2011, 50, 7179–7182;
- 28cH. Braunschweig, P. Brenner, R. D. Dewhurst, I. Krummenacher, B. Pfaffinger, A. Vargas, Nat. Commun. 2012, 3, 872.
- 29Calculated Wiberg bond indices are consistent with the observed bond lengths, see Supporting Information for details.
- 30See Supporting information for the calculated partial charges for 8 g and C8 g. The localization of negative charge on the carbon atom neighboring the boron atom is also observed for the 1,2-azaborine system, see: A. J. V. Marwitz, M. H. Matus, L. N. Zakharov, D. A. Dixon, S.-Y. Liu, Angew. Chem. 2009, 121, 991–995;
10.1002/ange.200805554 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 973–977.
- 31See Supporting Information for details.
- 32
- 32aY. Matsumoto, M. Naito, T. Hayashi, Organometallics 1992, 11, 2732–2734;
- 32bY. Matsumoto, M. Naito, Y. Uozumi, T. Hayashi, J. Chem. Soc. Chem. Commun. 1993, 1468–1469;
- 32cT. Hayashi, Acc. Chem. Res. 2000, 33, 354–362.
- 33The reaction of 8 h with [Pd2dba3] at room temperature for two hours resulted in the formation of a complex with a 11B NMR chemical shift of δ=32.0 ppm, an upfield shift of over 14 ppm from the corresponding resonance signal for the free ligand. The upfield shift in the 11B NMR resonance signal is consistent with our observation in the complexation of 8 g with platinum and indicates the formation of κ2-N-η2-BC complex.