Silylative Cyclopropanation of Allyl Phosphates with Silylboronates†
Corresponding Author
Dr. Ryo Shintani
Department of Chemistry and Biotechnology, Graduate School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)
Department of Chemistry and Biotechnology, Graduate School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)Search for more papers by this authorRyuhei Fujie
Department of Chemistry and Biotechnology, Graduate School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)
Search for more papers by this authorDr. Momotaro Takeda
Department of Chemistry, Graduate School of Science, Kyoto University, Kyoto 606-8502 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Kyoko Nozaki
Department of Chemistry and Biotechnology, Graduate School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)
Department of Chemistry and Biotechnology, Graduate School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)Search for more papers by this authorCorresponding Author
Dr. Ryo Shintani
Department of Chemistry and Biotechnology, Graduate School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)
Department of Chemistry and Biotechnology, Graduate School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)Search for more papers by this authorRyuhei Fujie
Department of Chemistry and Biotechnology, Graduate School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)
Search for more papers by this authorDr. Momotaro Takeda
Department of Chemistry, Graduate School of Science, Kyoto University, Kyoto 606-8502 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Kyoko Nozaki
Department of Chemistry and Biotechnology, Graduate School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)
Department of Chemistry and Biotechnology, Graduate School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)Search for more papers by this authorSupport has been provided in part by a Grant-in-Aid for Young Scientists (B), the Ministry of Education, Culture, Sports, Science and Technology (Japan), and the Asahi Glass Foundation. M.T. thanks JSPS for a fellowship.
Graphical Abstract
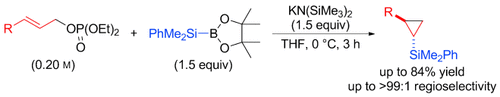
β attack! A potassium-bis(trimethylsilyl)amide-mediated cyclopropanation of allyl phosphates with silylboronates has been developed. Unlike the reported copper-catalyzed allylic substitution reactions, the nucleophile selectively attacks at the β-position of the allylic substrates under the present conditions. The reaction mechanism has also been investigated, thus indicating the involvement of a silylpotassium species as the active nucleophilic component.
Abstract
A potassium-bis(trimethylsilyl)amide-mediated cyclopropanation of allyl phosphates with silylboronates has been developed. Unlike the reported copper-catalyzed allylic substitution reactions, the nucleophile selectively attacks at the β-position of the allylic substrates under the present reaction conditions. The mechanism of this process has also been investigated, thus indicating the involvement of a silylpotassium species as the active nucleophilic component.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201403726_sm_miscellaneous_information.pdf4.3 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews, see:
- 1aH. Pellissier, Tetrahedron 2008, 64, 7041;
- 1bH. Lebel, J.-F. Marcoux, C. Molinaro, A. B. Charette, Chem. Rev. 2003, 103, 977.
- 2For selected examples, see:
- 2aS. Torii, H. Tanaka, Y. Nagai, Bull. Chem. Soc. Jpn. 1977, 50, 2825;
- 2bR. Verhé, N. De Kimpe, L. De Buyck, D. Courtheyn, L. Van Caenegem, N. Schamp, Bull. Soc. Chim. Belg. 1983, 92, 371;
- 2cM. P. Cooke, Jr., J. Y. Jaw, J. Org. Chem. 1986, 51, 758;
- 2dE. Vilsmaier, R. Adam, C. Tetzlaff, R. Cronauer, Tetrahedron 1989, 45, 3683;
- 2eS. Nakamura, Y. Watanabe, T. Toru, J. Chem. Soc. Perkin Trans. 1 1999, 3403;
- 2fH.-S. Jeon, S. Koo, Tetrahedron Lett. 2004, 45, 7023;
- 2gT. den Hartog, A. Rudolph, B. Marciá, A. J. Minnaard, B. L. Feringa, J. Am. Chem. Soc. 2010, 132, 14349.
- 3
- 3aL. S. Hegedus, W. H. Darlington, C. E. Russell, J. Org. Chem. 1980, 45, 5193;
- 3bH. M. R. Hoffmann, A. R. Otte, A. Wilde, Angew. Chem. 1992, 104, 224; Angew. Chem. Int. Ed. Engl. 1992, 31, 234;
- 3cA. Wilde, A. R. Otte, H. M. R. Hoffmann, J. Chem. Soc. Chem. Commun. 1993, 615;
- 3dA. R. Otte, A. Wilde, H. M. R. Hoffmann, Angew. Chem. 1994, 106, 1352; Angew. Chem. Int. Ed. Engl. 1994, 33, 1280;
- 3eH. M. R. Hoffmann, A. R. Otte, A. Wilde, S. Menzer, D. J. Williams, Angew. Chem. 1995, 107, 73;
10.1002/ange.19951070113 Google ScholarAngew. Chem. Int. Ed. Engl. 1995, 34, 100.
- 4
- 4aM. Formica, A. Musco, R. Pontellini, K. Linn, C. Mealli, J. Organomet. Chem. 1993, 448, C 6;
- 4bA. Satake, T. Nakata, J. Am. Chem. Soc. 1998, 120, 10391;
- 4cA. Satake, H. Koshino, T. Nakata, Chem. Lett. 1999, 49;
- 4dA. Satake, H. Kadohama, H. Koshino, T. Nakata, Tetrahedron Lett. 1999, 40, 3597;
- 4eW. Liu, D. Chen, X.-Z. Zhu, X.-L. Wan, X.-L. Hou, J. Am. Chem. Soc. 2009, 131, 8734.
- 5
- 5aR. Grigg, M. Kordes, Eur. J. Org. Chem. 2001, 707;
- 5bR. Shintani, S. Park, F. Shirozu, M. Murakami, T. Hayashi, J. Am. Chem. Soc. 2008, 130, 16174;
- 5cR. Shintani, T. Tsuji, S. Park, T. Hayashi, J. Am. Chem. Soc. 2010, 132, 7508;
- 5dR. Shintani, K. Moriya, T. Hayashi, Chem. Commun. 2011, 47, 3057;
- 5eR. Shintani, T. Ito, M. Nagamoto, H. Otomo, T. Hayashi, Chem. Commun. 2012, 48, 9936.
- 6R. Shintani, T. Ito, T. Hayashi, Org. Lett. 2012, 14, 2410.
- 7C. Zhong, S. Kunii, Y. Kosaka, M. Sawamura, H. Ito, J. Am. Chem. Soc. 2010, 132, 11440.
- 8A. Kawachi, H. Maeda, H. Nakamura, N. Doi, K. Tamao, J. Am. Chem. Soc. 2001, 123, 3143.
- 9
- 9aL. B. Delvos, D. J. Vyas, M. Oestreich, Angew. Chem. 2013, 125, 4748;
10.1002/ange.201300648 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 4650;
- 9bM. Takeda, R. Shintani, T. Hayashi, J. Org. Chem. 2013, 78, 5007.
- 10For recent reviews on SiB compounds, see:
- 10aM. Oestreich, E. Hartmann, M. Mewald, Chem. Rev. 2013, 113, 402;
- 10bT. Ohmura, M. Suginome, Bull. Chem. Soc. Jpn. 2009, 82, 29.
- 11For pioneering work on the preparation and use of this silylboronate, see:
- 11aM. Suginome, H. Nakamura, Y. Ito, Chem. Commun. 1996, 2777;
- 11bM. Suginome, T. Matsuda, Y. Ito, Organometallics 2000, 19, 4647.
- 12For selected recent examples for the transition-metal-catalyzed reactions using silylboronates, see:
- 12aT. Ohmura, K. Masuda, M. Suginome, J. Am. Chem. Soc. 2008, 130, 1526;
- 12bC. Walter, M. Oestreich, Angew. Chem. 2008, 120, 3878; Angew. Chem. Int. Ed. 2008, 47, 3818;
- 12cK. Lee, A. H. Hoveyda, J. Am. Chem. Soc. 2010, 132, 2898;
- 12dT. Ohmura, K. Oshima, H. Taniguchi, M. Suginome, J. Am. Chem. Soc. 2010, 132, 12194;
- 12eE. Hartmann, M. Oestreich, Angew. Chem. 2010, 122, 6331; Angew. Chem. Int. Ed. 2010, 49, 6195;
- 12fD. J. Vyas, M. Oestreich, Angew. Chem. 2010, 122, 8692;
10.1002/ange.201004658 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 8513;
- 12gP. Wang, X.-L. Yeo, T.-P. Loh, J. Am. Chem. Soc. 2011, 133, 1254;
- 12hK. Oshima, T. Ohmura, M. Suginome, J. Am. Chem. Soc. 2011, 133, 7324;
- 12iC. Kleeberg, M. S. Cheung, Z. Lin, T. B. Marder, J. Am. Chem. Soc. 2011, 133, 19060;
- 12jN. Saito, A. Kobayashi, Y. Sato, Angew. Chem. 2012, 124, 1254; Angew. Chem. Int. Ed. 2012, 51, 1228;
- 12kT. Fujihara, Y. Tani, K. Semba, J. Terao, Y. Tsuji, Angew. Chem. 2012, 124, 11655;
10.1002/ange.201207148 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 11487;
- 12lV. Cirriez, C. Rasson, T. Hermant, J. Petrignet, J. Díaz Álvarez, K. Robeyns, O. Riant, Angew. Chem. 2013, 125, 1829;
10.1002/ange.201209020 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 1785;
- 12mC. Zarate, R. Martin, J. Am. Chem. Soc. 2014, 136, 2236.
- 13For examples of the usage of silylboronates in the absence of transition-metal catalysts, see:
- 13aA. Kawachi, T. Minamimoto, K. Tamao, Chem. Lett. 2001, 1216;
- 13bJ. M. O’Brien, A. H. Hoveyda, J. Am. Chem. Soc. 2011, 133, 7712;
- 13cH. Ito, Y. Horita, E. Yamamoto, Chem. Commun. 2012, 48, 8006;
- 13dE. Yamamoto, K. Izumi, Y. Horita, H. Ito, J. Am. Chem. Soc. 2012, 134, 19997.
- 14Addition of silylboronates to styrenes catalyzed by KOtBu has been reported in Ref. [13c].
- 15ICP-AES analysis of KN(SiMe3)2 (Aldrich, 95 %) showed that detectable trace metals were Na (480 ppm), Mg (2.7 ppm), Ca (5.6 ppm), Fe (0.7 ppm), and Zr (0.2 ppm) where 1 ppm=1×10−6 mg per 1 mg of KN(SiMe3)2.
- 16Notes:
- 16aThe substrates 1 bearing a heteroaryl group such as R=2-furyl or 1-methyl-6-indolyl could not be employed because of their thermal instability.
- 16bThe substrates 1 bearing an alkyl group such as R=n-pentyl were not applicable to the present cyclopropanation, resulting in the exclusive formation of the allylic substitution products 4.
- 17Y. Cui, W. Li, T. Sato, Y. Yamashita, S. Kobayashi, Adv. Synth. Catal. 2013, 355, 1193.
- 18Generation and spectroscopic confirmation of a silyllithium by the reaction of a silylboronate with MeLi or nBuLi have been reported in Ref. [13a]. Similarly, generation of a silylpotassium from a silylboronate and excess KOtBu and a silylmagnesium bromide from a silylboronate and excess MeMgBr have also been suggested (with no spectroscopic evidence) in this paper.
- 19E. Buncel, T. K. Venkatachalam, B. Eliasson, U. Edlund, J. Am. Chem. Soc. 1985, 107, 303.
- 20We currently do not have a good explanation for the origin of the selective nucleophilic attack at the β-position. For relevant examples using silicon nucleophiles, see Refs. [8] and [13c].
- 21For a review, see: G. R. Jones, Y. Landais, Tetrahedron 1996, 52, 7599.
- 22aK. Tamao, N. Ishida, T. Tanaka, M. Kumada, Organometallics 1983, 2, 1694;
- 22bK. Tamao, N. Ishida, J. Organomet. Chem. 1984, 269, C 37;
- 22cK. Tamao, E. Nakajo, Y. Ito, J. Org. Chem. 1987, 52, 4412;
- 22dK. Tamao in Organosilicon and Bioorganosilicon Chemistry (Ed.: ), Ellis Horwood, Chichester, 1985, p. 231.
- 23
- 23aI. Fleming, R. Henning, H. Plaut, J. Chem. Soc. Chem. Commun. 1984, 29;
- 23bI. Fleming, P. E. J. Sanderson, Tetrahedron Lett. 1987, 28, 4229.
- 24For a related example of the migration of a phenyl group from silicon to carbon, see Ref. [23a].
- 25For a review on the cleavage of aryl–silicon bonds, see: C. Eaborn, J. Organomet. Chem. 1975, 100, 43.
- 26This was confirmed by using [D2]-5 a (obtained from [D2]-1 a) for the ring-opening oxidation reaction. See the Supporting Information for details.