Metal-Free Annulation of Arenes with 2-Aminopyridine Derivatives: The Methyl Group as a Traceless Non-Chelating Directing Group†
Correction(s) for this article
-
Corrigendum: Metal-Free Annulation of Arenes with 2-Aminopyridine Derivatives: The Methyl Group as a Traceless Non-Chelating Directing Group
- Volume 53Issue 37Angewandte Chemie International Edition
- pages: 9695-9695
- First Published online: September 2, 2014
Srimanta Manna
Abteilung Chemische Biologie, Max-Planck-Institut für Molekulare Physiologie, Otto-Hahn-Strasse 11, 44227 Dortmund (Germany)
Fakultät Chemie und Chemische Biologie, Technische Universität Dortmund, Otto-Hahn-Strasse 6, 44221 Dortmund (Germany)
Search for more papers by this authorDr. Kiran Matcha
Abteilung Chemische Biologie, Max-Planck-Institut für Molekulare Physiologie, Otto-Hahn-Strasse 11, 44227 Dortmund (Germany)
Search for more papers by this authorCorresponding Author
Dr. Andrey P. Antonchick
Abteilung Chemische Biologie, Max-Planck-Institut für Molekulare Physiologie, Otto-Hahn-Strasse 11, 44227 Dortmund (Germany)
Fakultät Chemie und Chemische Biologie, Technische Universität Dortmund, Otto-Hahn-Strasse 6, 44221 Dortmund (Germany)
Abteilung Chemische Biologie, Max-Planck-Institut für Molekulare Physiologie, Otto-Hahn-Strasse 11, 44227 Dortmund (Germany)===Search for more papers by this authorSrimanta Manna
Abteilung Chemische Biologie, Max-Planck-Institut für Molekulare Physiologie, Otto-Hahn-Strasse 11, 44227 Dortmund (Germany)
Fakultät Chemie und Chemische Biologie, Technische Universität Dortmund, Otto-Hahn-Strasse 6, 44221 Dortmund (Germany)
Search for more papers by this authorDr. Kiran Matcha
Abteilung Chemische Biologie, Max-Planck-Institut für Molekulare Physiologie, Otto-Hahn-Strasse 11, 44227 Dortmund (Germany)
Search for more papers by this authorCorresponding Author
Dr. Andrey P. Antonchick
Abteilung Chemische Biologie, Max-Planck-Institut für Molekulare Physiologie, Otto-Hahn-Strasse 11, 44227 Dortmund (Germany)
Fakultät Chemie und Chemische Biologie, Technische Universität Dortmund, Otto-Hahn-Strasse 6, 44221 Dortmund (Germany)
Abteilung Chemische Biologie, Max-Planck-Institut für Molekulare Physiologie, Otto-Hahn-Strasse 11, 44227 Dortmund (Germany)===Search for more papers by this authorWe thank Prof. Dr. Herbert Waldmann for his generous support.
Graphical Abstract
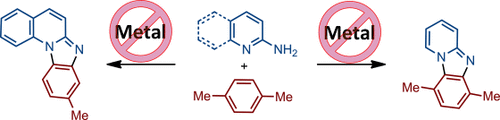
Disappearing Me: A novel selective annulation between 2-aminopyridine derivatives and arenes under metal-free conditions provides the important pyrido[1,2-a]benzimidazole scaffold under mild reaction conditions. In this intermolecular reaction the methyl group of methylbenzenes serves as a traceless, non-chelating, and highly regioselective directing group.
Abstract
A novel annulation reaction between 2-aminopyridine derivatives and arenes under metal-free conditions is described. The presented intermolecular transformation provided straightforward access to the important pyrido[1,2-a]benzimidazole scaffold under mild reaction conditions. The unprecedented application of the methyl group of methylbenzenes as a traceless, non-chelating, and highly regioselective directing group is reported.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201403712_sm_miscellaneous_information.pdf1.5 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For medicinal properties of benzimidazole compounds, see:
- 1aR. S. Begunov, G. A. Ryzvanovicha, Russ. Chem. Rev. 2013, 82, 77–97;
- 1bH. Takeshita, J. Watanabe, Y. Kimura, K. Kawakami, H. Takahashi, M. Takemura, A. Kitamura, K. Someya, R. Nakajima, Bioorg. Med. Chem. Lett. 2010, 20, 3893–3896;
- 1cM. Sedic, M. Poznic, P. Gehrig, M. Scott, R. Schlapbach, M. Hranjec, G. Karminski-Zamola, K. Pavelic, S. K. Pavelic, Mol. Cancer Ther. 2008, 7, 2121–2132;
- 1dM. Hranjec, I. Piantanida, M. Kralj, L. Šuman, K. i. Pavelić, G. Karminski-Zamola, J. Med. Chem. 2008, 51, 4899–4910;
- 1eM. Hranjec, M. Kralj, I. Piantanida, M. Sedić, L. Šuman, K. Pavelić, G. Karminski-Zamola, J. Med. Chem. 2007, 50, 5696–5711;
- 1fS. K. Kotovskaya, Z. M. Baskakova, V. N. Charushin, O. N. Chupakhin, E. F. Belanov, N. I. Bormotov, S. M. Balakhnin, O. A. Serova, Pharm. Chem. J. 2005, 39, 574–578.
- 2
- 2aJ. A. Adachi, H. L. DuPont, Clin. Infect. Dis. 2006, 42, 541–547;
- 2bE. Marchi, G. Mascellani, L. Montecchi, A. P. Venturini, M. Brufani, L. Cellai, J. Med. Chem. 1985, 28, 960–963.
- 3For the synthesis of pyrido[1,2-a]benzimidazoles through C–H amination, see:
- 3aY. He, J. Huang, D. Liang, L. Liu, Q. Zhu, Chem. Commun. 2013, 49, 7352–7354;
- 3bD. Liang, Y. He, L. Liu, Q. Zhu, Org. Lett. 2013, 15, 3476–3479;
- 3cN. Kutsumura, S. Kunimatsu, K. Kagawa, T. Otani, T. Saito, Synthesis 2011, 3235–3240;
- 3dH. Wang, Y. Wang, C. Peng, J. Zhang, Q. Zhu, J. Am. Chem. Soc. 2010, 132, 13217–13219.
- 4For other synthesis methods for pyrido[1,2-a]benzimidazoles, see:
- 4aZ. Wu, Q. Huang, X. Zhou, L. Yu, Z. Li, D. Wu, Eur. J. Org. Chem. 2011, 5242–5245;
- 4bK. Noriki, K. Shinichi, K. Kimiko, O. Takashi, S. Takao, Synthesis 2011, 3235–3240;
- 4cD. Zhao, J. Hu, N. Wu, X. Huang, X. Qin, J. Lan, J. You, Org. Lett. 2011, 13, 6516–6519;
- 4dC. G. Yan, Q. F. Wang, X. K. Song, J. Sun, J. Org. Chem. 2009, 74, 710–718;
- 4eC. Venkatesh, G. S. M. Sundaram, H. Ila, H. Junjappa, J. Org. Chem. 2006, 71, 1280–1283;
- 4fG. Morgan, S. Stewart, J. Chem. Soc. 1939, 1057–1066;
- 4gG. Morgan, S. Stewart, J. Chem. Soc. 1938, 1292–1305.
- 5For reviews on C–H amination, see:
- 5aM. L. Louillat, F. W. Patureau, Chem. Soc. Rev. 2014, 43, 901–910;
- 5bR. Samanta, A. P. Antonchick, Synlett 2012, 809–813;
- 5cF. Collet, C. Lescot, P. Dauban, Chem. Soc. Rev. 2011, 40, 1926–1936;
- 5dF. Collet, R. H. Dodd, P. Dauban, Chem. Commun. 2009, 5061–5074.
- 6For reviews on the chemistry of IIII compounds, see:
- 6aV. V. Zhdankin, Hypervalent Iodine Chemistry: Preparation, Structure and Synthetic Applications of Polyvalent Iodine Compounds, Wiley, Chichester, 2014;
- 6bM. S. Yusubov, V. V. Zhdankin, Curr. Org. Synth. 2012, 9, 247–272;
- 6cJ. L. F. Silva, Jr., B. Olofsson, Nat. Prod. Rep. 2011, 28, 1722–1754;
- 6dE. A. Merritt, B. Olofsson, Synthesis 2011, 517–538;
- 6eJ. P. Brand, D. F. Gonzalez, S. Nicolai, J. Waser, Chem. Commun. 2011, 47, 102–115;
- 6fV. V. Zhdankin, ARKIVOC 2009, 1–62;
- 6gM. Uyanik, K. Ishihara, Chem. Commun. 2009, 2086–2099;
- 6hT. Dohi, M. Ito, N. Yamaoka, K. Morimoto, H. Fujioka, Y. Kita, Tetrahedron 2009, 65, 10797–10815;
- 6iV. V. Zhdankin, P. J. Stang, Chem. Rev. 2008, 108, 5299–5358;
- 6jS. Quideau, L. Pouysegu, D. Deffieux, Synlett 2008, 467–495.
- 7For selected C–H amination reactions using IIII reagents, see:
- 7aS. K. Alla, R. K. Kumar, P. Sadhu, T. Punniyamurthy, Org. Lett. 2013, 15, 1334–1337;
- 7bG. Jacquemot, M.-A. Menard, C. L. Homme, S. Canesi, Chem. Sci. 2013, 4, 1287–1292;
- 7cC. Röben, J. A. Souto, E. C. Escudero-Adán, K. Muñiz, Org. Lett. 2013, 15, 1008–1011;
- 7dJ. A. Souto, D. Zian, K. Muñiz, J. Am. Chem. Soc. 2012, 134, 7242–7245;
- 7eX. Ban, Y. Pan, Y. Lin, S. Wang, Y. Du, K. Zhao, Org. Biomol. Chem. 2012, 10, 3606–3609;
- 7fU. Farid, T. Wirth, Angew. Chem. 2012, 124, 3518–3522;
10.1002/ange.201107703 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 3462–3465;
- 7gY. Du, R. Liu, G. Linn, K. Zhao, Org. Lett. 2006, 8, 5919–5922;
- 7hH. J. Kim, S. H. Cho, S. Chang, Org. Lett. 2012, 14, 1424–1427;
- 7iA. A. Kantak, S. Potavathri, R. A. Barham, K. M. Romano, B. DeBoef, J. Am. Chem. Soc. 2011, 133, 19960–19965;
- 7jH. J. Kim, J. Kim, S. H. Cho, S. Chang, J. Am. Chem. Soc. 2011, 133, 16382–16385.
- 8For a review on the work of our group, see:
- 8aR. Samanta, K. Matcha, A. P. Antonchick, Eur. J. Org. Chem. 2013, 5769–5804; and for recent work, see:
- 8bR. Samanta, J. O. Bauer, C. Strohmann, A. P. Antonchick, Org. Lett. 2012, 14, 5518–5521;
- 8cR. Samanta, J. Lategahn, A. P. Antonchick, Chem. Commun. 2012, 48, 3194–3196;
- 8dA. P. Antonchick, R. Samanta, K. Kulikov, J. Lategahn, Angew. Chem. 2011, 123, 8764–8767;
10.1002/ange.201102984 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 8605–8608.
- 9For leading reviews on coupling without chelating, see:
- 9aN. Kuhl, M. N. Hopkinson, J. Wencel-Delord, F. Glorius, Angew. Chem. 2012, 124, 10382–10401;
10.1002/ange.201203269 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 10236–10254;
- 9bJ. A. Ashenhurst, Chem. Soc. Rev. 2010, 39, 540–548.
- 10For reviews on the development of traceless directing groups, see:
- 10aG. Rousseau, B. Breit, Angew. Chem. 2011, 123, 2498–2543;
10.1002/ange.201006139 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 2450–2494; for selected recent works, see:
- 10bJ. M. Neely, T. Rovis, J. Am. Chem. Soc. 2014, 136, 2735–2738;
- 10cJ. Luo, S. Preciado, I. Larrosa, J. Am. Chem. Soc. 2014, 136, 4109–4112;
- 10dC. Wang, Y. Huang, Org. Lett. 2013, 15, 5294–5297;
- 10eS. M. Preshlock, D. L. Plattner, P. E. Maligres, S. W. Krska, R. E. Maleczka, M. R. Smith, Angew. Chem. 2013, 125, 13153–13157;
10.1002/ange.201306511 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 12915–12919;
- 10fX. Huang, J. Huang, C. Du, X. Zhang, F. Song, J. You, Angew. Chem. 2013, 125, 13208–13212; Angew. Chem. Int. Ed. 2013, 52, 12970–12974;
- 10gA. V. Gulevich, F. S. Melkonyan, D. Sarkar, V. Gevorgyan, J. Am. Chem. Soc. 2012, 134, 5528–5531;
- 10hL. Ackermann, E. Diers, A. Manvar, Org. Lett. 2012, 14, 1154–1157;
- 10iR. Samanta, A. P. Antonchick, Angew. Chem. 2011, 123, 5323–5326; Angew. Chem. Int. Ed. 2011, 50, 5217–5220.
- 11
- 11aD. Sharma, C. B. Reddy, A. K. Shil, R. P. Saroach, P. Das, Mol. Diversity 2013, 17, 651–659;
- 11bA. Jha, ARKIVOC 2006, 13–20;
- 11cC. Wu, J. Org. Chem. 1998, 63, 2348–2350;
- 11dR. A. Olofson, J. T. Martz, J. P. Senet, M. Piteau, T. Malfroot, J. Org. Chem. 1984, 49, 2081–2082.