Total Synthesis and Stereochemical Reassignment of Mandelalide A†
Honghui Lei
Laboratory of Chemical Genomics, School of Chemical Biology and Biotechnology, Peking University Shenzhen Graduate School, Xili, Nanshan District, Shenzhen, 518055 (China)
Search for more papers by this authorJialei Yan
Laboratory of Chemical Genomics, School of Chemical Biology and Biotechnology, Peking University Shenzhen Graduate School, Xili, Nanshan District, Shenzhen, 518055 (China)
Search for more papers by this authorJie Yu
Laboratory of Chemical Genomics, School of Chemical Biology and Biotechnology, Peking University Shenzhen Graduate School, Xili, Nanshan District, Shenzhen, 518055 (China)
Search for more papers by this authorDr. Yuqing Liu
Department of Applied Biology & Chemical Technology, The Hong Kong Polytechnic University, Kowloon, Hong Kong (China)
Search for more papers by this authorZhuo Wang
Department of Applied Biology & Chemical Technology, The Hong Kong Polytechnic University, Kowloon, Hong Kong (China)
Search for more papers by this authorCorresponding Author
Prof. Zhengshuang Xu
Laboratory of Chemical Genomics, School of Chemical Biology and Biotechnology, Peking University Shenzhen Graduate School, Xili, Nanshan District, Shenzhen, 518055 (China)
Zhengshuang Xu, Laboratory of Chemical Genomics, School of Chemical Biology and Biotechnology, Peking University Shenzhen Graduate School, Xili, Nanshan District, Shenzhen, 518055 (China)
Tao Ye, Department of Applied Biology & Chemical Technology, The Hong Kong Polytechnic University, Kowloon, Hong Kong (China)
Search for more papers by this authorCorresponding Author
Prof. Tao Ye
Department of Applied Biology & Chemical Technology, The Hong Kong Polytechnic University, Kowloon, Hong Kong (China)
Zhengshuang Xu, Laboratory of Chemical Genomics, School of Chemical Biology and Biotechnology, Peking University Shenzhen Graduate School, Xili, Nanshan District, Shenzhen, 518055 (China)
Tao Ye, Department of Applied Biology & Chemical Technology, The Hong Kong Polytechnic University, Kowloon, Hong Kong (China)
Search for more papers by this authorHonghui Lei
Laboratory of Chemical Genomics, School of Chemical Biology and Biotechnology, Peking University Shenzhen Graduate School, Xili, Nanshan District, Shenzhen, 518055 (China)
Search for more papers by this authorJialei Yan
Laboratory of Chemical Genomics, School of Chemical Biology and Biotechnology, Peking University Shenzhen Graduate School, Xili, Nanshan District, Shenzhen, 518055 (China)
Search for more papers by this authorJie Yu
Laboratory of Chemical Genomics, School of Chemical Biology and Biotechnology, Peking University Shenzhen Graduate School, Xili, Nanshan District, Shenzhen, 518055 (China)
Search for more papers by this authorDr. Yuqing Liu
Department of Applied Biology & Chemical Technology, The Hong Kong Polytechnic University, Kowloon, Hong Kong (China)
Search for more papers by this authorZhuo Wang
Department of Applied Biology & Chemical Technology, The Hong Kong Polytechnic University, Kowloon, Hong Kong (China)
Search for more papers by this authorCorresponding Author
Prof. Zhengshuang Xu
Laboratory of Chemical Genomics, School of Chemical Biology and Biotechnology, Peking University Shenzhen Graduate School, Xili, Nanshan District, Shenzhen, 518055 (China)
Zhengshuang Xu, Laboratory of Chemical Genomics, School of Chemical Biology and Biotechnology, Peking University Shenzhen Graduate School, Xili, Nanshan District, Shenzhen, 518055 (China)
Tao Ye, Department of Applied Biology & Chemical Technology, The Hong Kong Polytechnic University, Kowloon, Hong Kong (China)
Search for more papers by this authorCorresponding Author
Prof. Tao Ye
Department of Applied Biology & Chemical Technology, The Hong Kong Polytechnic University, Kowloon, Hong Kong (China)
Zhengshuang Xu, Laboratory of Chemical Genomics, School of Chemical Biology and Biotechnology, Peking University Shenzhen Graduate School, Xili, Nanshan District, Shenzhen, 518055 (China)
Tao Ye, Department of Applied Biology & Chemical Technology, The Hong Kong Polytechnic University, Kowloon, Hong Kong (China)
Search for more papers by this authorWe acknowledge financial support from the Hong Kong Research Grants Council (PolyU 5040/10P, PolyU 5037/11P, PolyU 5020/12P, PolyU5030/13P, and PolyU153035/14P), the Fong Shu Fook Tong Foundation, the Joyce M. Kuok Foundation, the National Natural Science Foundation of China (21072007, 21272011, and 21133002), and the Shenzhen Science and Technology Development Fund (JCYJ20130329175740481 and ZYC201105170351A).
Graphical Abstract
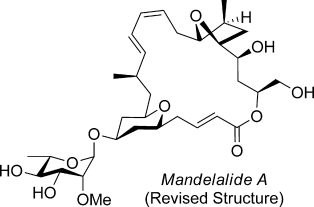
Structural revision: A revised configurational assignment for the marine macrolide mandelalide A is proposed and validated by total synthesis. This study is one of several recent examples in a growing list of investigations that correct misassigned structures of natural products by stereocontrolled total synthesis.
Abstract
The total synthesis of the tunicate metabolite mandelalide A and the correction of its originally assigned stereochemistry are reported. Key features of the convergent, fully stereocontrolled route include the use of a Prins cyclization for the diastereoselective construction of the tetrahydropyran subunit, Rychnovsky–Bartlett cyclization for the preparation of the tetrahydrofuran moiety, Suzuki coupling, Horner–Wadsworth–Emmons macrocyclization, and glycosylation to append the L-rhamnose-derived pyranoside.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201403542_sm_miscellaneous_information.pdf4.2 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J. Sikorska, A. M. Hau, C. Anklin, S. Parker-Nance, M. T. Davies-Coleman, J. E. Ishmael, K. L. McPhail, J. Org. Chem. 2012, 77, 6066.
- 2
- 2aL. Song, J. Liu, H. Gui, C. Hui, J. Zhou, Y. Guo, P. Zhang, Z. Xu, T. Ye, Chem. Asian J. 2013, 8, 2955;
- 2bH. Liu, Y. Liu, Z. Wang, X. Xing, A. R. Maguire, H. Luesch, H. Zhang, Z. Xu, T. Ye, Chem. Eur. J. 2013, 19, 6774;
- 2cL. Dai, B. Chen, H. Lei, Z. Wang, Y. Liu, Z. Xu, T. Ye, Chem. Commun. 2012, 48, 8697;
- 2dS. Li, Z. Chen, Z. Xu, T. Ye, Chem. Commun. 2010, 46, 4773;
- 2eZ. Chen, L. Song, Z. Xu, T. Ye, Org. Lett. 2010, 12, 2036;
- 2fB. Chen, L. Dai, H. Zhang, W. Tan, Z. Xu, T. Ye, Chem. Commun. 2010, 46, 574.
- 3H. Pellissier, Tetrahedron 2005, 61, 2947.
- 4J. A. Bisceglia, L. R. Orelli, Curr. Org. Chem. 2012, 16, 2206.
- 5S. D. Rychnovsky, P. A. Bartlett, J. Am. Chem. Soc. 1981, 103, 3964.
- 6
- 6aX. Han, G. Peh, P. E. Floreancig, Eur. J. Org. Chem. 2013, 1193;
- 6bI. M. Pastor, M. Yus, Curr. Org. Chem. 2012, 16, 1277.
- 7A. B. Smith III, G. K. Friestad, J. Barbosa, E. Bertounesque, K. G. Hull, M. Iwashima, Y. Qiu, B. A. Salvatore, P. G. Spoors, J. J.-W. Duan, J. Am. Chem. Soc. 1999, 121, 10468.
- 8
- 8aL. A. Paquette, S. K. Chang, Org. Lett. 2005, 7, 3111;
- 8bC. Nilewski, N. R. Deprez, T. C. Fessard, D. B. Li, R. W. Geisser, E. M. Carreira, Angew. Chem. 2011, 123, 8087;
10.1002/ange.201102521 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 7940.
- 9
- 9aP. R. Blakemore, W. J. Cole, P. J. Kocienski, A. Morley, Synlett 1998, 26;
- 9bP. R. Blakemore, J. Chem. Soc. Perkin Trans. 1 2002, 2563.
- 10L. Zhu, D. R. Mootoo, Org. Lett. 2003, 5, 3475.
- 11P. A. Bartlett, P. C. Ting, J. Org. Chem. 1986, 51, 2230.
- 12H. Furuta, Y. Hasegawa, M. Hase, Y. Mori, Chem. Eur. J. 2010, 16, 7586.
- 13G. Stock, K. Zhao, Tetrahedron 1989, 30, 2173.
- 14H. C. Kolb, M. S. VanNieuwenhze, K. B. Sharpless, Chem. Rev. 1994, 94, 2483.
- 15For detailed assignment of the stereochemistry of the newly formed secondary alcohol 21, see the Supporting Information.
- 16
- 16aJ. Inanaga, K. Hirata, H. Saeki, T. Katsuki, M. Yamaguchi, Bull. Chem. Soc. Jpn. 1979, 52, 1989;
- 16bM. Hikota, Y. Sakurai, K. Horita, O. Yonemitsu, Tetrahedron Lett. 1990, 31, 6367.
- 17F. Keyling-Bilger, G. Schmitt, A. Beck, B. Luu, Tetrahedron 1996, 52, 14891.
- 18F. Fu, T.-P. Loh, Tetrahedron Lett. 2009, 50, 3530.
- 19The mechanism of the racemization in the Prins cyclization reactions has been elucidated; see: R. Jasti, S. D. Rychnovsky, J. Am. Chem. Soc. 2006, 128, 13640.
- 20S. Müller, B. Liepold, G. J. Roth, H. J. Bestmann, Synlett 1996, 521.
- 21K. Shirakawa, A. Arase, M. Hoshi, Synthesis 2004, 1814.
- 22M. Fragoso-Serrano, G. Guillén-Jaramillo, R. Pereda-Miranda, C. M. Cerda-García-Rojas, J. Org. Chem. 2003, 68, 7167.
- 23S. V. Ley, H. W. M. Priepke, S. L. Warrine, Angew. Chem. Int. Ed. Engl. 1994, 33, 2290; Angew. Chem. 1994, 106, 2410.
- 24
- 24aS. V. Ley, D. R. Owen, K. E. Wesson, J. Chem. Soc. Perkin Trans. 1 1997, 2805;
- 24bA. Cirla, A. R. McHale, J. Mann, Tetrahedron 2004, 60, 4019.
- 25D. Kahne, S. Walker, Y. Cheng, D. Van Engen, J. Am. Chem. Soc. 1989, 111, 6881.
- 26A. De Mico, R. Margarita, L. Parlanti, A. Vescovi, G. Piancatelli, J. Org. Chem. 1997, 62, 6974.
- 27M. A. Blanchette, W. Choy, J. T. Davis, A. P. Essenfeld, S. Masamune, W. R. Roush, T. Sakai, Tetrahedron Lett. 1984, 25, 2183.
- 28H. Wehlan, M. Dauber, M. T. M. Fernaud, J. Schuppan, S. Keiper, R. Mahrwald, M.-E. J. Garcia, U. Koert, Chem. Eur. J. 2006, 12, 7378.
- 29J. Willwacher, A. Fürstner, Angew. Chem. Int. Ed. 2014, 53, 4217; Angew. Chem. 2014, 126, 4301.
- 30See the Supporting Information.
- 31
- 31aP. L. Winder, Ph.D. Thesis, Florida Atlantic University, 2009;
- 31bI. Paterson, G. W. Haslett, Org. Lett. 2013, 15, 1338.
- 32For an initial cytotoxicity evaluation of the synthetic compounds, see the Supporting Information.