Reversible Redox Reaction Between Antiaromatic and Aromatic States of 32π-Expanded Isophlorins†
We are grateful to IISER Pune and CSIR, New Delhi, India for the financial support. T.Y.G. thanks CSIR, New Delhi, India, for a senior research fellowship.
Graphical Abstract
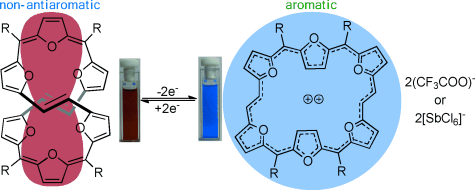
Pi redox: Expanded isophlorins can undergo reversible two-electron redox reactions to interconvert between 32π-antiaromatic and 30π-aromatic states. trifluoroacetic acid, Et3O+SbCl6−, or NOBF4 and triethylamine, zinc, or FeCl2 can be employed as oxidizing and reducing agents, respectively. This reversible redox process can also regulate the topology between a figure-eight and planar conformation.
Abstract
32π-antiaromatic expanded isophlorins with a varying number of thiophene and furan rings adopt either planar, ring-inverted, or twisted conformations depending on the number of furan rings in the macrocycle. However, they exhibit identical reactivity with respect to their oxidation to aromatic 30π-dicationic species under acidic conditions. These 32π-antiaromatic macrocycles can also be oxidized with [Et3O+SbCl6−]and NOBF4 to generate dications, thus confirming ring oxidation of macrocycles. Furthermore, they can be reduced back to their parent 32π-antiaromatic state by triethylamine, Zn, or FeCl2. Single-crystal X-ray diffraction analysis confirmed a figure-eight conformation for a hexafuran system, which opens to a planar structure upon oxidation.