Metal–Organic Frameworks—The New Jack of All Trades for (Inorganic) Chemists
Graphical Abstract
“…︁ Today, the research contexts in which the work on MOFs are, or will be placed, are enormously diverse and multilingual, and the technical backgrounds and motives of those involved develop equally as diversely, sometimes with a very distant reference to the original. Therefore, distinctions and care in the use of scientific language and expression are important. …︁” Read more in the Editorial by Roland A. Fischer.
What does the term metal–organic framework (MOF) mean to you? Do you have a spontaneous picture that comes to mind? I have to admit that I for one didn’t really have one, and in fact probably had a blind spot in my perception, until I noticed, while leafing through Angewandte Chemie in 2004, an article that opened my eyes, namely the Review “Functional Porous Coordination polymers” by Susumu Kitagawa et al. on page 2388. The discussion is about zeolite-like solids that exceed all known materials in terms of porosity by orders of magnitude, while offering fascinating new dimensions of molecular design with enormous internal surfaces and spaces. Possible applications therefore include not only energy storage (for example in form of hydrogen and methane) or the catalysis, but also biomedicine, sensors, microelectronics, and more. MOFs form when inorganic and organic building blocks are combined to build coordination compounds with network structures. The art of well-planned synthesis of network compounds (reticular structures) has become very sophisticated. In the last decade alone, more than 20 000 MOFs have been described, as can be seen from a graph (Figure 1) from the publication by O. M. Yaghi et al. (Science, 2013, 341, 974), and there are hardly any issues of Angewandte Chemie in which something new about MOFs cannot be found. International MOF conferences have been held regularly since 2008, such as this year in Kobe (28th September to 1st October). The term “metal–organic framework” delivers around 3.6 million hits in a Google search, where as “zeolite” results “only” in 1.5 million, and the article by S. Kitagawa mentioned above has just this year become the most cited article in Angewandte Chemie to date.1

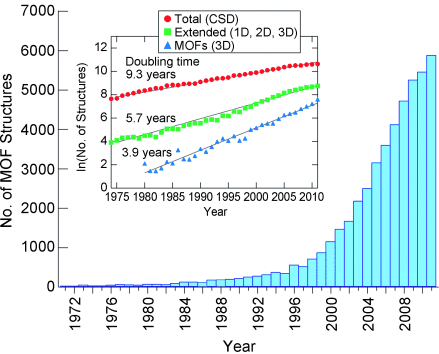
Metal–organic frameworks (1D, 2D, and 3D) that were deposited in the Cambridge Structural Database 1971–2011. The largest increase so far has been in 3D MOFs with a doubling of the documented structures every four years.
So much for the relevance of the now well-established term. However, how should we judge its value in regards of rigorous scientific terminology? What would a precise definition, which meaningfully fits into the overall context of coordination chemistry, look like? The IUPAC working group “Coordination Polymers and Metal–Organic Frameworks: Terminology and Nomenclature Guidelines” documented and analyzed the language use (English) from 2009 and recently submitted its final report (Pure Appl. Chem. 2013, 85, 1715–1724), the translation of which will appear shortly in Angewandte Chemie.
The authors recommend a hierarchical terminology, starting from the coordination polymer as a generic term, whereby coordination compounds with network structures are a subset of coordination polymers, and metal–organic frameworks, in turn, are a subset of the coordination compounds with network structures. The use of the classification as organic–inorganic hybrid compounds is discouraged.
The proposed definition is: “A metal–organic framework compound is a coordination compound with organic ligands and potential cavities.” What is potential porosity? The structures of many of these coordination compounds are dynamic and therefore change, and so eventually do the cavities under the influence of external stimuli and/or as a result of the storage or release of guest molecules. Since this responsiveness of the material is one of its most charming and probably unique properties, rigid crystalline order and permanent (constant) porosity are not considered as a necessary criterion for a MOF. Interesting, isn’t it?
With this suggestion to read the original text (see also CrystEngComm 2012, 14, 3001–3004), and keeping in mind the authority of the IUPAC Working Group, has everything essential already been said on the topic? In the strict sense, this could well be answered in the affirmative, because part and parcel of science are the discovery and coinage of terminology, differentiation, utilization practices, critical-discursive reflection, and the placement of the new into the inventory of the old and familiar, which leads to clarifications, (more or less preliminary) definitions, and differentiation of the terms. Remarkably, the IUPAC Working Group emphasizes however, it is aware that there are two mutually exclusive views among the experts: one view is that conceptually “MOF” is meaningfully valid only for carboxylate compounds, and the other is that the new name is superfluous and should not be used at all. This discussion could plausibly have been held ten years ago, and would have then perhaps prevented the inconsistent use of the terminology in many thousands of scientific articles.
I use the tension insinuated here as an occasion for a final comment: The term metal–organic framework had emerged sporadically already in the early 1990s. Its coinage and establishment as MOF, however, is very closely linked to Omar M. Yaghi and Michael O’Keeffe, and at the latest in 1999, with the publication on MOF-5, [Zn4O(bdc)3] (bdc=1,4-benzenedicarboxylate), entitled “Design and synthesis of an exceptionally stable and highly porous metal–organic framework” (Nature 1999, 402, 276), the fascinating perspectives of the concept and its serious possibility of realization became clear. Thus, the term MOF demands (or demanded) originality and vision. Because the technical terminology of the family name of the new substances is obscure, it can foster imagination, conceptual interest, and curiosity beyond the chemical technical or foreign language, where “metal”, “organic”, and “framework” are certainly more suitable than “coordination”, “polymer”, and “network.” Language and its use are creative, and the Babylonian confusion since the Tower of Babel, reinterpreted as a gift, perhaps deems, not least, to inspiring diversity and the pursuit of understanding; it is a mission to productively overcome misunderstandings.
Today, the research contexts in which the work on MOFs are, or will be placed, are enormously diverse and multilingual, and the technical backgrounds and motives of those involved develop equally as diversely, sometimes with a very distant reference to the original. Therefore, distinctions and care in the use of scientific language and expression are important. Which MOFs can be brought together in a meaningful relationship? Which conceptual aspects of research on and with a variety of MOFs are transferable to other MOFs and/or (porous) coordination compounds and polymers? What is the significance of constitution and topology, specific structure–property relationships, and functions of MOFs as a criterion of demarcation? Answers to such questions are relevant. However, they can be only inadequately derived from rules of scientific nomenclature and also not from a precise translation of accepted technical terms and definitions from English into German or into another language. Rather, they are in each individual case a matter of convincing argumentation: The importance of a paper on a coordination polymer is hardly magnified by the fact that the material may appropriately be declared a MOF or, to emphasize the creation of a new material named XYZ-123 or equipped with a prefix or new acronym. If the content of the term MOF is reduced too much to constitutional and structural features, whether in narrower or broader terms, it loses essential aspects of its original context. Associated with this is the idea to understand highly porous coordination compounds as an attractive and challenging synthetic target in the transition field of molecular and solid-state chemistry, to think in terms of network topologies, and in view of interesting supramolecular chemical and physical features to build, modify, and understand robust crystalline materials in a controlled and precise manner. In this respect, the question of whether one can claim to be talking about a metal–organic framework compound in a specific case remains fundamentally exciting for me.





