Polymerase Synthesis of Photocaged DNA Resistant against Cleavage by Restriction Endonucleases†
Zuzana Vaníková
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Gilead Sciences & IOCB Research Center, Flemingovo nám. 2, 16610 Prague 6 (Czech Republic) http://www.uochb.cas.cz/hocekgroup
Search for more papers by this authorCorresponding Author
Prof. Dr. Michal Hocek
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Gilead Sciences & IOCB Research Center, Flemingovo nám. 2, 16610 Prague 6 (Czech Republic) http://www.uochb.cas.cz/hocekgroup
Department of Organic Chemistry, Faculty of Science, Charles University in Prague, Hlavova 8, 12843 Prague 2 (Czech Republic)
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Gilead Sciences & IOCB Research Center, Flemingovo nám. 2, 16610 Prague 6 (Czech Republic) http://www.uochb.cas.cz/hocekgroupSearch for more papers by this authorZuzana Vaníková
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Gilead Sciences & IOCB Research Center, Flemingovo nám. 2, 16610 Prague 6 (Czech Republic) http://www.uochb.cas.cz/hocekgroup
Search for more papers by this authorCorresponding Author
Prof. Dr. Michal Hocek
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Gilead Sciences & IOCB Research Center, Flemingovo nám. 2, 16610 Prague 6 (Czech Republic) http://www.uochb.cas.cz/hocekgroup
Department of Organic Chemistry, Faculty of Science, Charles University in Prague, Hlavova 8, 12843 Prague 2 (Czech Republic)
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Gilead Sciences & IOCB Research Center, Flemingovo nám. 2, 16610 Prague 6 (Czech Republic) http://www.uochb.cas.cz/hocekgroupSearch for more papers by this authorThis work was supported by the ASCR (RVO: 61388963) and by the Czech Science Foundation (14-04289S). The authors thank Dr. R. Pohl for NMR analyses and Prof. P. Klán for help with photochemical experiments.
Graphical Abstract
Abstract
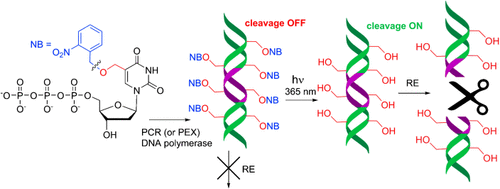
5-[(2-Nitrobenzyl)oxymethyl]-2′-deoxyuridine 5′-O-triphosphate was used for polymerase (primer extension or PCR) synthesis of photocaged DNA that is resistant to the cleavage by restriction endonucleases. Photodeprotection of the caged DNA released 5-hydroxymethyluracil-modified nucleic acids, which were fully recognized and cleaved by restriction enzymes.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201402370_sm_miscellaneous_information.pdf2.4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Reviews:
- 1aP. Klán, T. Solomek, C. G. Bochet, A. Blanc, R. Givens, M. Rubina, V. Popik, A. Kostikov, J. Wirz, Chem. Rev. 2013, 113, 119–191;
- 1bI. Ahmed, L. Fruk, Mol. Biosyst. 2013, 9, 565–570.
- 2Reviews:
- 2aQ. Liu, A. Deiters, Acc. Chem. Res. 2014, 47, 45–55;
- 2bX. Tang, J. Zhang, J. Sun, Y. Wang, J. Wu, L. Zhang, Org. Biomol. Chem. 2013, 11, 7814–7824;
- 2cL. M. Ceo, J. T. Koh, ChemBioChem 2012, 13, 511–513;
- 2dJ. P. Casey, R. A. Blinder, W. T. Monroe, Mol. Pharm. 2009, 6, 669–685.
- 3Examples of base-photocaged nucleic acids:
- 3aA. Deiters, R. A. Garner, H. Lusic, J. M. Goven, M. Dush, N. M. Nascone-Yoder, J. A. Yoder, J. Am. Chem. Soc. 2010, 132, 15644–15650;
- 3bJ. M. Govan, R. Uprety, J. Hemphill, M. O. Lively, A. Deiters, ACS Chem. Biol. 2012, 7, 1247–1256.
- 4Examples of phosphate-photocaged nucleic acids:
- 4aW. T. Monroe, M. M. McQuain, M. S. Chang, J. S. Alexander, F. R. Haselton, J. Biol. Chem. 1999, 274, 20895–20900;
- 4bS. Shah, S. Rangarajan, S. H. Friedman, Angew. Chem. 2005, 117, 1352–1356;
10.1002/ange.200461458 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 1328–1332;
- 4cA. Teraoka, K. Murakoshi, K. Fukamauchi, A. Z. Suzuki, S. Watanabe, T. Furuta, Chem. Commun. 2014, 50, 664–666.
- 5J. Wu, X. Tang, Bioorg. Med. Chem. 2013, 21, 6205–6211.
- 6
- 6aM. J. E. Resendiz, A. Schon, E. Freire, M. M. Greenberg, J. Am. Chem. Soc. 2012, 134, 12478–12481;
- 6bJ. M. N. San Pedro, M. M. Greenberg, ChemBioChem 2013, 14, 1590–1596.
- 7
- 7aV. A. Litosh, W. D. Wu, B. P. Stupi, J. C. Wang, S. E. Morris, M. N. Hersh, M. L. Metzker, Nucleic Acids Res. 2011, 39, e 39;
- 7bB. P. Stupi, H. Li, J. C. Wang, W. D. Wu, S. E. Morris, V. A. Litosh, J. Muniz, M. N. Hersh, M. L. Metzker, Angew. Chem. 2012, 124, 1756–1759;
10.1002/ange.201106516 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 1724–1727.
- 8
- 8aH. Macíčková-Cahová, M. Hocek, Nucleic Acids Res. 2009, 37, 7612–7622;
- 8bH. Macíčková-Cahová, R. Pohl, M. Hocek, ChemBioChem 2011, 12, 431–438.
- 9P. Kielkowski, H. Macíčková-Cahová, R. Pohl, M. Hocek, Angew. Chem. 2011, 123, 8886–8889;
10.1002/ange.201102898 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 8727–8730.
- 10P. Kielkowski, N. L. Brock, J. S. Dickschat, M. Hocek, ChemBioChem 2013, 14, 801–804.
- 11
- 11aP. M. Rae, Science 1976, 194, 1062–1064;
- 11bJ. H. Gommers-Ampt, P. Borst, FASEB J. 1995, 9, 1034–1042;
- 11cF. Van Leeuwen, M. C. Taylor, A. Mondragon, H. Moreau, W. Gibson, R. Kieft, P. Borst, Proc. Natl. Acad. Sci. USA 1998, 95, 2366–2371.
- 12T. Pfaffeneder, F. Spada, M. Wagner, C. Brandmayr, S. Laube, D. Eisen, M. Truss, J. Steinbacher, B. Hackner, O. Kotljarova, D. Schuermann, S. Michalakis, O. Kosmatchev, S. Schiesser, B. Steigenberger, N. Raddaoui, G. Kashiwazaki, U. Müller, C. G. Spruijt, M. Vermeulen, H. Leonhardt, P. Schär, M. Müller, T. Carell, Nat. Chem. Biol. 2014, DOI: .
- 13Reviews:
- 13aT. Carell, C. Brandmayr, A. Hienzsch, M. Muller, D. Pearson, V. Reiter, I. Thoma, P. Thumbs, M. Eagner, Angew. Chem. 2012, 124, 7220–7242;
10.1002/ange.201201193 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 7110–7131;
- 13bM. Münzel, D. Globisch, T. Carell, Angew. Chem. 2011, 123, 6588–6596; Angew. Chem. Int. Ed. 2011, 50, 6460–6468;
- 13cY. Fu, C. He, Curr. Opin. Chem. Biol. 2012, 16, 516–524.
- 14L. Lercher, M. A. McDonough, A. H. El-Sagheer, A. Thalhammer, S. Kriaucionis, T. Brown, C. J. Schofield, Chem. Commun. 2014, 50, 1794–1796.
- 15Related papers on the synthesis of protected UHM nucleosides:
- 15aQ. Dai, C.-X. Song, T. Pan, C. He, J. Org. Chem. 2011, 76, 4182–4188;
- 15bA. S. Hansen, A. Thalhammer, A. H. El-Sagheer, T. Brown, C. J. Schofield, Bioorg. Med. Chem. Lett. 2011, 21, 1181–1184;
- 15cL. C. Sowers, G. P. Beardsley, J. Org. Chem. 1993, 58, 1664–1665;
- 15dG. T. Shiau, R. F. Schinazi, M. S. Chen, W. H. Prusoff, J. Med. Chem. 1980, 23, 127–133;
- 15eM. Münzel, D. Globish, T. Brückl, M. Wagner, V. Welzmiller, S. Michalakis, M. Müller, M. Biel, T. Carell, Angew. Chem. 2010, 122, 5503–5505; Angew. Chem. Int. Ed. 2010, 49, 5375–5377.
- 16E. P. Quinlivan, J. F. Gregory III, Anal. Biochem. 2008, 373, 383–385.