Asymmetric Catalysis on the Nanoscale: The Organocatalytic Approach to Helicenes†
Lisa Kötzner
Max-Planck-Institut für Kohlenforschung, Kaiser Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany)
Search for more papers by this authorDr. Matthew J. Webber
Max-Planck-Institut für Kohlenforschung, Kaiser Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany)
Search for more papers by this authorDr. Alberto Martínez
Max-Planck-Institut für Kohlenforschung, Kaiser Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany)
Search for more papers by this authorDr. Claudia De Fusco
Max-Planck-Institut für Kohlenforschung, Kaiser Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Benjamin List
Max-Planck-Institut für Kohlenforschung, Kaiser Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany)
Max-Planck-Institut für Kohlenforschung, Kaiser Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany)===Search for more papers by this authorLisa Kötzner
Max-Planck-Institut für Kohlenforschung, Kaiser Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany)
Search for more papers by this authorDr. Matthew J. Webber
Max-Planck-Institut für Kohlenforschung, Kaiser Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany)
Search for more papers by this authorDr. Alberto Martínez
Max-Planck-Institut für Kohlenforschung, Kaiser Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany)
Search for more papers by this authorDr. Claudia De Fusco
Max-Planck-Institut für Kohlenforschung, Kaiser Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Benjamin List
Max-Planck-Institut für Kohlenforschung, Kaiser Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany)
Max-Planck-Institut für Kohlenforschung, Kaiser Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany)===Search for more papers by this authorWe gratefully acknowledge generous support from the Max Planck Society, the European Research Council (Advanced Grant “High Performance Lewis Acid Organocatalysis, HIPOCAT”), and the Ministero dell′Istruzione, dell′Università e della Ricerca (MIUR) (fellowship for C.D.F.). We also thank the members of our HPLC, NMR, MS, and crystallography departments for their support.
Graphical Abstract
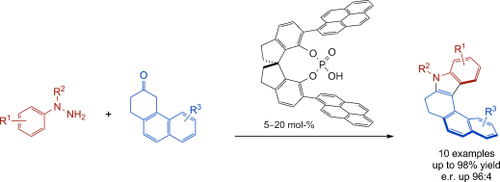
Twisting indoles: A novel chiral Brønsted acid, specifically designed for long-range control on a nanoscale, catalyzes the asymmetric synthesis of azahelicenes through a Fischer indolization. The method has the advantage of starting from simple achiral starting materials, which can be modified by changing the protecting group (R2) or the terminal substituents (R1, R3). The products can be further oxidized to polyaromatic systems.
Abstract
The first asymmetric organocatalytic synthesis of helicenes is reported. A novel SPINOL-derived phosphoric acid, bearing extended π-substituents, catalyzes the asymmetric synthesis of helicenes through an enantioselective Fischer indole reaction. A variety of azahelicenes and diazahelicenes could be obtained with good to excellent yields and enantioselectivities.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201400474_sm_miscellaneous_information.pdf4.2 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews on the synthesis and applications of helicenes, see
- 1aM. Gingras, Chem. Soc. Rev. 2013, 42, 968–1006;
- 1bM. Gingras, G. Félix, R. Peresutti, Chem. Soc. Rev. 2013, 42, 1007–1050;
- 1cM. Gingras, Chem. Soc. Rev. 2013, 42, 1051–1095;
- 1dY. Shen, C.-F. Chen, Chem. Rev. 2012, 112, 1463–1535;
- 1eI. G. Stará, I. Starý in Science of Synthesis, Vol. 45b (Eds.: ), Thieme, Stuttgart, 2010, pp. 885–953;
- 1fI. Starý, I. G. Stará in Strained Hydrocarbons (Ed.: ), Wiley-VCH, Weinheim, 2009, pp. 166–176;
- 1gA. Rajca, M. Miyasaka in Functional Organic Materials (Eds.: ), Wiley-VCH, Weinheim, 2007, pp. 547–581;
- 1hA. Urbano, Angew. Chem. 2003, 115, 4116–4119;
10.1002/ange.200301667 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 3986–3989.
- 2
- 2aA. Grandbois, S. K. Collins, Chem. Eur. J. 2008, 14, 9323–9329;
- 2bA. Sudhakar, T. Katz, J. Am. Chem. Soc. 1986, 108, 179–181;
- 2cM. C. Carreño, S. García-Cerrada, A. Urbano, J. Am. Chem. Soc. 2001, 123, 7929–7930;
- 2dY. Ogawa, M. Toyama, M. Karikomi, K. Seki, K. Haga, T. Uyehara, Tetrahedron Lett. 2003, 44, 2167–2170;
- 2eM. C. Carreño, M. González-López, A. Urbano, Chem. Commun. 2005, 611–613;
- 2fI. G. Stará, Z. Alexandrová, F. Teplý, P. Sehnal, I. Starý, D. Šaman, M. Budĕšínský, J. Cvačka, Org. Lett. 2005, 7, 2547–2550;
- 2gP. Sehnal, I. G. Stará, D. Šaman, M. Tichý, J. Míšek, J. Cvačka, L. Rulíšek, J. Chocholoušova, J. Vacek, G. Goryl, M. Szymonski, I. Císařová, I. Starý, Proc. Natl. Acad. Sci. USA 2009, 106, 13169–13174;
- 2hM. S. M. Pearson, D. R. Carbery, J. Org. Chem. 2009, 74, 5320–5325;
- 2iJ. Žádný, A. Jančařík, A. Andronova, M. Šámal, J. V. Chocholoušová, J. Vacek, R. Pohl, D. Šaman, I. Císařová, I. G. Stará, I. Starý, Angew. Chem. 2012, 124, 5959–5963;
10.1002/ange.201108307 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 5857–5861.
- 3For selected examples, see
- 3aI. G. Stará, I. Starý, A. Kollárovic, F. Teplý, Š. Vyskočil, D. Šaman, Tetrahedron Lett. 1999, 40, 1993–1996;
- 3bF. Teplý, I. G. Stará, I. Starý, A. Kollárovic, D. Šaman, Š. Vyskočil, P. Fiedler, J. Org. Chem. 2003, 68, 5193–5197;
- 3cJ. Caeiro, D. Peña, A. Cobas, D. Pérez, E. Guitián, Adv. Synth. Catal. 2006, 348, 2466–2474;
- 3dK. Tanaka, A. Kamisawa, T. Suda, K. Noguchi, M. Hirano, J. Am. Chem. Soc. 2007, 129, 12078–12079;
- 3eK. Tanaka, N. Fukawa, T. Suda, K. Noguchi, Angew. Chem. 2009, 121, 5578–5581; Angew. Chem. Int. Ed. 2009, 48, 5470–5473;
- 3fN. Fukawa, T. Osaka, K. Noguchi, K. Tanaka, Org. Lett. 2010, 12, 1324–1327;
- 3gT. Shibata, T. Uchiyama, Y. Yoshinami, S. Takayasu, K. Tsuchikama, K. Endo, Chem. Commun. 2012, 48, 1311–1313;
- 3hY. Sawada, S. Furumi, A. Takai, M. Takeuchi, K. Noguchi, K. Tanaka, J. Am. Chem. Soc. 2012, 134, 4080–4083;
- 3iA. Jančařík, J. Rybáček, K. Cocq, J. Vacek Chocholoušová, J. Vacek, R. Pohl, L. Bednárová, P. Fiedler, I. Císařová, I. G. Stará, I. Starý, Angew. Chem. 2013, 125, 10154–10159;
10.1002/ange.201301739 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 9970–9975.
- 4
- 4aS. Müller, M. J. Webber, B. List, J. Am. Chem. Soc. 2011, 133, 18534–18537;
- 4bA. Martínez, M. J. Webber, S. Müller, B. List, Angew. Chem. 2013, 125, 9664–9668; Angew. Chem. Int. Ed. 2013, 52, 9486–9490.
- 5
- 5aJ. Meisenheimer, K. Witte, Ber. Dtsch. Chem. Ges. 1903, 36, 4153–4164;
- 5bW. Fuchs, F. Niszel, Ber. Dtsch. Chem. Ges. 1927, 60, 209–217;
- 5cF. B. Mallory, C. S. Wood, J. T. Gordon, J. Am. Chem. Soc. 1964, 86, 3094–3102;
- 5dI. Pischel, S. Grimme, S. Kotila, M. Nieger, F. Vögtle, Tetrahedron: Asymmetry 1996, 7, 109–116;
- 5eS. D. Dreher, D. J. Weix, T. J. Katz, J. Org. Chem. 1999, 64, 3671–3678;
- 5fK. Nakano, Y. Hidehira, K. Takahashi, T. Hiyama, K. Nozaki, Angew. Chem. 2005, 117, 7298–7300; Angew. Chem. Int. Ed. 2005, 44, 7136–7138.
- 6For the original mechanistic proposal, see G. M. Robinson, R. Robinson, J. Chem. Soc. Abstr. 1924, 125, 827–840.
- 7Studies on the nonlinear effects are in agreement with our concept, with no nonlinear effect observed for our reaction (see the Supporting Information).
- 8For reviews on chiral phosphoric acid catalysis, see
- 8aM. Terada, Synthesis 2010, 1929–1982;
- 8bD. Kampen, C. M. Reisinger, B. List, Top. Curr. Chem. 2010, 291, 395–456;
- 8cT. Akiyama, Chem. Rev. 2007, 107, 5744–5758.
- 9SPINOL-derived phosphoric acids were independently introduced by three research groups:
- 9aF. Xu, D. Huang, C. Han, W. Shen, X. Lin, Y. Wang, J. Org. Chem. 2010, 75, 8677–8680;
- 9bI. Čorić, S. Müller, B. List, J. Am. Chem. Soc. 2010, 132, 17370–17373;
- 9cC.-H. Xing, Y.-X. Liao, J. Ng, Q.-S. Hu, J. Org. Chem. 2011, 76, 4125–4131.
- 10The corresponding doubly benzylated compound was only formed in trace amounts in the presence of catalyst (S)-5 e.
- 11The fully debenzylated compound was not detected by MS.
- 12
- 12aM. S. Newnam, R. S. Darlak, L. Tsai, J. Am. Chem. Soc. 1967, 89, 6191–6193;
- 12bD. A. Lightner, D. T. Hefelfinger, T. W. Powers, G. W. Frank, K. N. Trueblood, J. Am. Chem. Soc. 1972, 94, 3492–3497.
- 13
- 13aR. H. Martin, M. J. Marchant, Tetrahedron 1974, 30, 347–349;
- 13bR. H. Martin, M. J. Marchant, Tetrahedron Lett. 1972, 13, 3707–3708; for computational studies, see
10.1016/S0040-4039(01)94141-3 Google Scholar
- 13cR. H. Janke, G. Haufe, E.-U. Würthwein, J. H. Borkent, J. Am. Chem. Soc. 1996, 118, 6031–6035.
- 14Compound 3 a decomposed at 240 °C, so its corresponding racemization data were not consistent.