In Situ Generation of Diimide from Hydrazine and Oxygen: Continuous-Flow Transfer Hydrogenation of Olefins†
Bartholomäus Pieber
Christian Doppler Laboratory for Microwave Chemistry (CDLMC) and Institute of Chemistry, Karl-Franzens-University Graz, Heinrichstrasse 28, 8010 Graz (Austria) http://www.maos.net
Search for more papers by this authorDr. Sabrina Teixeira Martinez
Christian Doppler Laboratory for Microwave Chemistry (CDLMC) and Institute of Chemistry, Karl-Franzens-University Graz, Heinrichstrasse 28, 8010 Graz (Austria) http://www.maos.net
Search for more papers by this authorDr. David Cantillo
Christian Doppler Laboratory for Microwave Chemistry (CDLMC) and Institute of Chemistry, Karl-Franzens-University Graz, Heinrichstrasse 28, 8010 Graz (Austria) http://www.maos.net
Search for more papers by this authorCorresponding Author
Prof. C. Oliver Kappe
Christian Doppler Laboratory for Microwave Chemistry (CDLMC) and Institute of Chemistry, Karl-Franzens-University Graz, Heinrichstrasse 28, 8010 Graz (Austria) http://www.maos.net
Christian Doppler Laboratory for Microwave Chemistry (CDLMC) and Institute of Chemistry, Karl-Franzens-University Graz, Heinrichstrasse 28, 8010 Graz (Austria) http://www.maos.netSearch for more papers by this authorBartholomäus Pieber
Christian Doppler Laboratory for Microwave Chemistry (CDLMC) and Institute of Chemistry, Karl-Franzens-University Graz, Heinrichstrasse 28, 8010 Graz (Austria) http://www.maos.net
Search for more papers by this authorDr. Sabrina Teixeira Martinez
Christian Doppler Laboratory for Microwave Chemistry (CDLMC) and Institute of Chemistry, Karl-Franzens-University Graz, Heinrichstrasse 28, 8010 Graz (Austria) http://www.maos.net
Search for more papers by this authorDr. David Cantillo
Christian Doppler Laboratory for Microwave Chemistry (CDLMC) and Institute of Chemistry, Karl-Franzens-University Graz, Heinrichstrasse 28, 8010 Graz (Austria) http://www.maos.net
Search for more papers by this authorCorresponding Author
Prof. C. Oliver Kappe
Christian Doppler Laboratory for Microwave Chemistry (CDLMC) and Institute of Chemistry, Karl-Franzens-University Graz, Heinrichstrasse 28, 8010 Graz (Austria) http://www.maos.net
Christian Doppler Laboratory for Microwave Chemistry (CDLMC) and Institute of Chemistry, Karl-Franzens-University Graz, Heinrichstrasse 28, 8010 Graz (Austria) http://www.maos.netSearch for more papers by this authorThis work was supported by a grant from the Christian Doppler Research Society (CDG). We gratefully thank Prof. Walter Goessler for performing ICP-MS measurements. S.T.N. thanks CNPq for a fellowship. D.C. thanks the Research, Technological Innovation, and Supercomputing Center of Extremadura (CénitS) for their support in the use of LUSITANIA computer resources.
Graphical Abstract
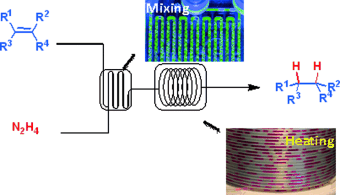
No catalyst required! A highly efficient, catalyst-free process to generate diimide in situ from hydrazine monohydrate and molecular oxygen for the selective reduction of alkenes has been developed. The use of a gas–liquid segmented flow system allowed safe operating conditions and dramatically enhanced this atom-economical reaction, resulting in short processing times.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201303528_sm_miscellaneous_information.pdf732.6 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Alternative names for diimide reported in the literature are diazene and diimine.
- 2
- 2aS. Hünig, H. R. Müller, W. Thier, Angew. Chem. 1965, 77, 368; Angew. Chem. Int. Ed. Engl. 1965, 4, 271;
- 2bD. J. Pasto, R. T. Taylor, Org. React. 1991, 40, 91.
- 3D. Sellmann, A. Hennige, Angew. Chem. 1997, 109, 270;
10.1002/ange.19971090321 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 276.
- 4
- 4aP. A. Chaloner, M. A. Esteruleas, F. Joó, L. A. Oro, Homogeneous Hydrogenation, Kluwer, Dodrecht, 1994;
10.1007/978-94-017-1791-5 Google Scholar
- 4b Handbook of Homogeneous Hydrogenation, Vols. 1–3 (Eds.: ), Wiley-VCH, Weinheim, 2007.
- 5M. Lamani, G. S. Ravikumara, K. R. Prabhu, Adv. Synth. Catal. 2012, 354, 1437.
- 6For reports on olefin reductions under argon atmosphere employing N2H4 in the presence of Fe3O4 nanoparticles or Rh–Fe3O4 heterodimer nanocrystals without further oxidizing agents, see:
- 6aE. Kim, S. Kim, B. M. Kim, Bull. Korean Chem. Soc. 2011, 32, 3183;
- 6bY. Jang, S. Kim, S. W. Jun, B. H. Kim, S. Hwang, I. K. Song, B. M. Kim, T. Hyeon, Chem. Commun. 2011, 47, 3601.
- 7M. Lamani, R. S. Guralamata, K. R. Prabhu, Chem. Commun. 2012, 48, 6583.
- 8
- 8aY. Imada, H. Iida, T. Naota, J. Am. Chem. Soc. 2005, 127, 14544;
- 8bC. Smit, M. W. Fraaije, A. J. Minnaard, J. Org. Chem. 2008, 73, 9482;
- 8cB. J. Marsh, E. L. Heath, D. R. Carbery, Chem. Commun. 2011, 47, 280;
- 8dJ. F. Teichert, T. den Hartog, M. Hanstein, C. Smit, B. ter Horst, V. Hernandez-Olmos, B. L. Feringa, A. J. Minnaard, ACS Catal. 2011, 1, 309;
- 8eY. Imada, H. Iida, T. Kitagawa, T. Naota, Chem. Eur. J. 2011, 17, 5908.
- 9M. P. Feth, K. Rossen, A. Burgard, Org. Process Res. Dev. 2013, 17, 282.
- 10 Micro Process Engineering (Eds.: ), Wiley-Blackwell, Oxford, 2009.
- 11For a recent summary and further information about gas–liquid flow chemistry, see: T. Noël, V. Hessel, ChemSusChem 2013, 6, 405; and references cited therein.
- 12For a recent publication describing olefin reductions in continuous-flow mode using diimide generated by a different pathway from hydroxylamine and N,O-bistrifluoroacetyl hydroxylamine, see: A. S. Kleinke, T. F. Jamison, Org. Lett. 2013, 15, 710.
- 13For a recent review on novel process windows and process-intensification technologies based on high-throughput conditions, see: V. Hessel, D. Kralisch, N. Kockmann, T. Noël, Q. Wang, ChemSusChem 2013, 6, 746.
- 14A detailed description of the continuous-flow setup is given in the Supporting Information; see also: B. Gutmann, D. Obermayer, J.-P. Roduit, D. M. Roberge, C. O. Kappe, J. Flow Chem. 2012, 1, 8.
- 15R. Battino, T. R. Rettich, T. Tominaga, J. Phys. Chem. Ref. Data 1983, 12, 163.
- 16The only by-product observed in these experiments, according to GC-MS analysis, was a small amount of 1,2-dipropylidenehydrazine resulting from solvent oxidation and subsequent reaction with hydrazine (Figure S5).
- 17Caution: The reactions/reagents described herein have the potential to release large amounts of energy in an uncontrolled way. These oxidations should not be undertaken without stringent hazard assessment and proper safety precautions in place.
- 18M. Hamano, K. D. Nagy, K. F. Jensen, Chem. Commun. 2012, 48, 2086.
- 19For further details on the calculations and references, see the Supporting Information.
- 20This remarkable selectivity is the result of performing the reaction in the absence of a metal catalyst. For an Fe-catalyzed, hydrazine-mediated method for nitro-group reduction, see: D. Cantillo, M. Baghbanzadeh, C. O. Kappe, Angew. Chem. 2012, 124, 10337;
10.1002/ange.201205792 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 10190.
- 21H. Sajiki, Tetrahedron Lett. 1995, 36, 3465.