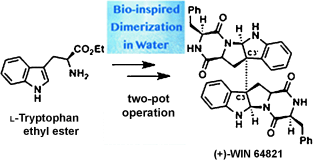
Bio-Inspired Dimerization Reaction of Tryptophan Derivatives in Aqueous Acidic Media: Three-Step Syntheses of (+)-WIN 64821, (−)-Ditryptophenaline, and (+)-Naseseazine B†
Shinji Tadano
Department of Chemistry, Graduate School of Science and Technology, Kumamoto University, 2-39-1, Kurokami, Chuo-ku, Kumamoto 860-8555 (Japan) http://www.sci.kumamoto-u.ac.jp/∼ishikawa/ishikawa-lab/Top.html
Search for more papers by this authorYuri Mukaeda
Department of Chemistry, Graduate School of Science and Technology, Kumamoto University, 2-39-1, Kurokami, Chuo-ku, Kumamoto 860-8555 (Japan) http://www.sci.kumamoto-u.ac.jp/∼ishikawa/ishikawa-lab/Top.html
Search for more papers by this authorCorresponding Author
Prof. Dr. Hayato Ishikawa
Department of Chemistry, Graduate School of Science and Technology, Kumamoto University, 2-39-1, Kurokami, Chuo-ku, Kumamoto 860-8555 (Japan) http://www.sci.kumamoto-u.ac.jp/∼ishikawa/ishikawa-lab/Top.html
Department of Chemistry, Graduate School of Science and Technology, Kumamoto University, 2-39-1, Kurokami, Chuo-ku, Kumamoto 860-8555 (Japan) http://www.sci.kumamoto-u.ac.jp/∼ishikawa/ishikawa-lab/Top.htmlSearch for more papers by this authorShinji Tadano
Department of Chemistry, Graduate School of Science and Technology, Kumamoto University, 2-39-1, Kurokami, Chuo-ku, Kumamoto 860-8555 (Japan) http://www.sci.kumamoto-u.ac.jp/∼ishikawa/ishikawa-lab/Top.html
Search for more papers by this authorYuri Mukaeda
Department of Chemistry, Graduate School of Science and Technology, Kumamoto University, 2-39-1, Kurokami, Chuo-ku, Kumamoto 860-8555 (Japan) http://www.sci.kumamoto-u.ac.jp/∼ishikawa/ishikawa-lab/Top.html
Search for more papers by this authorCorresponding Author
Prof. Dr. Hayato Ishikawa
Department of Chemistry, Graduate School of Science and Technology, Kumamoto University, 2-39-1, Kurokami, Chuo-ku, Kumamoto 860-8555 (Japan) http://www.sci.kumamoto-u.ac.jp/∼ishikawa/ishikawa-lab/Top.html
Department of Chemistry, Graduate School of Science and Technology, Kumamoto University, 2-39-1, Kurokami, Chuo-ku, Kumamoto 860-8555 (Japan) http://www.sci.kumamoto-u.ac.jp/∼ishikawa/ishikawa-lab/Top.htmlSearch for more papers by this authorThis work was supported financially by a Grant-in-Aid for Scientific Research (B) (25288050) and Exploratory Research (25670005) from JSPS, a Grant-in-Aid for Scientific Research on Innovative Areas “Advanced Molecular Transformations by Organocatalysis” (No. 2304) (24105528) from MEXT, the Naito Foundation, the Uehara Memorial Foundation, the NOVARTIS Foundation (Japan) for the promotion of Science, a SUNBOR Grant from the Suntory Institute for Bioorganic Research, and the JGC-S Scholarship Foundation. We also thank Prof. Hiroshi Nishino (Kumamoto University), Prof. Ryo Irie (Kumamoto University), and Prof. Hiromitsu Takayama (Chiba University) for their support and guidance.
Graphical Abstract
Doubling up: The direct bio-inspired dimerization of commercially available amine-free tryptophan derivatives in aqueous acidic media provides C2-symmetrical and nonsymmetrical dimeric compounds. Further processing completes the concise syntheses of naturally occurring dimeric diketopiperazine alkaloids such as (+)-WIN 64821 (see picture) in overall yields of up to 20 %.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201303143_sm_miscellaneous_information.pdf25 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aG. A. Cordell, J. E. Saxton, The Alkaloids: Chemistry and Physiology, Vol. 20, Academic Press, New York, 1981, pp. 3–295;
- 1bT. Hino, M. Nakagawa in The Alkaloids: Chemistry and Pharmacology, Vol. 34 (Ed.: ), Academic Press, New York, 1989, chap. 1, pp. 1–75;
10.1016/S0099-9598(08)60226-6 Google Scholar
- 1c“Chemistry and Reactions of Cyclic Tautomers of Tryptamines and Tryptophans”: U. Anthoni, C. Christophersen, P. H. Nielsen in Alkaloids: Chemical and Biological Perspectives, Vol. 13 (Ed.: ), Pergamon, London, 1999, chap. 2, pp. 163–236.
10.1016/S0735-8210(99)80025-9 Google Scholar
- 2
- 2aC. J. Barrow, P. Cai, J. K. Snyder, D. M. Sedlock, H. H. Sun, R. Cooper, J. Org. Chem. 1993, 58, 6016–6021;
- 2bJ. L. Popp, L. L. Musza, C. J. Barrow, P. J. Rudewicz, D. R. Houck, J. Antibiot. 1994, 47, 411–419;
- 2cJ. J. Oleynek, D. M. Sedlock, C. J. Barrow, K. C. Appell, F. Casiano, D. Haycock, S. J. Ward, P. Kaplita, A. M. Gillum, J. Antibiot. 1994, 47, 399–410;
- 2dD. M. Sedlock, C. J. Barrow, J. E. Brownell, A. Hong, A. M. Gillum, P. R. Houck, J. Antibiot. 1994, 47, 391–398;
- 2eM. Hiramoto, M. Shibazaki, H. Miyata, Y. Saita, Tennen Yuki Kagobutsu Toronkai Koen Yoshishu 1994, 36, 557–569;
- 2fC. J. Barrow, L. L. Musza, R. Cooper, Bioorg. Med. Chem. Lett. 1995, 5, 377–380.
- 3R. Raju, A. M. Piggott, M. Conte, W. G. L. Aalbersberg, K. Feussner, R. J. Capon, Org. Lett. 2009, 11, 3862–3865.
- 4J. Kim, M. Movassaghi, J. Am. Chem. Soc. 2011, 133, 14940–14943.
- 5G. Ding, L. Jiang, L. Guo, X. Chen, H. Zhang, Y. Che, J. Nat. Prod. 2008, 71, 1861–1865.
- 6C. Pérez-Balado, Á. R. de Lera, Org. Biomol. Chem. 2010, 8, 5179–5186.
- 7S. Cai, X. Kong, W. Wang, H. Zhou, T. Zhu, D. Li, Q. Gu, Tetrahedron Lett. 2012, 53, 2615–2617.
- 8
- 8aM. Nakagawa, H. Sugumi, S. Kodato, T. Hino, Tetrahedron Lett. 1981, 22, 5323–5326;
- 8bL. E. Overman, D. V. Paone, J. Am. Chem. Soc. 2001, 123, 9465–9467;
- 8cS. P. Govek, L. E. Overman, Tetrahedron 2007, 63, 8499–8513;
- 8dC. Pérez-Balado, Á. R. de Lera, Org. Lett. 2008, 10, 3701–3704;
- 8eJ. Kim, J. A. Ashenhurst, M. Movassaghi, Science 2009, 324, 238–241;
- 8fC. Pérez-Balado, P. Rodríguez-Graña, Á. R. de Lera, Chem. Eur. J. 2009, 15, 9928–9937;
- 8gJ. Kim, M. Movassaghi, J. Am. Chem. Soc. 2010, 132, 14376–14378;
- 8hE. Iwasa, Y. Hamashima, S. Fujishiro, E. Higuchi, A. Ito, M. Yoshida, M. Sodeoka, J. Am. Chem. Soc. 2010, 132, 4078–4079;
- 8iJ. E. DeLorbe, S. Y. Jabri, S. M. Mennen, L. E. Overman, F.-L. Zhang, J. Am. Chem. Soc. 2011, 133, 6549–6552;
- 8jE. Iwasa, Y. Hamashima, S. Fujishiro, D. Hashizume, M. Sodeoka, Tetrahedron 2011, 67, 6587–6599;
- 8kN. Boyer, M. Movassaghi, Chem. Sci. 2012, 3, 1798–1803;
- 8lM. Sodeoka, K. Dodo, Y. Teng, K. Iuchi, Y. Hamashima, E. Iwasa, S. Fujishiro, Pure Appl. Chem. 2012, 84, 1369–1378;
- 8mM. E. Kieffer, K. V. Chuang, S. E. Reisman, J. Am. Chem. Soc. 2013, 135, 5557–5560; review see:
- 8nJ. Kim, M. Movassaghi, Chem. Soc. Rev. 2009, 38, 3035–3050.
- 9
- 9aM. Movassaghi, M. A. Schmidt, J. A. Ashenhurst, Angew. Chem. 2008, 120, 1507–1509;
10.1002/ange.200704960 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 1485–1487;
- 9bM. Movassaghi, O. K. Ahmad, S. P. Lathrop, J. Am. Chem. Soc. 2011, 133, 13002–13005.
- 10
- 10aH. Ishikawa, D. A. Colby, D. L. Boger, J. Am. Chem. Soc. 2008, 130, 420–421;
- 10bH. Ishikawa, D. A. Colby, S. Seto, V. P. A. Tam, H. Kakei, T. J. Rayl, I. Hwang, D. L. Boger, J. Am. Chem. Soc. 2009, 131, 4904–4916;
- 10cH. Gotoh, J. E. Sears, A. Eschenmoser, D. L. Boger, J. Am. Chem. Soc. 2012, 134, 13240–13243.
- 11
- 11aS. M. Kupchan, O. P. Dhingra, C.-K. Kim, V. Kameswaran, J. Org. Chem. 1978, 43, 2521–2529;
- 11bT. C. Jempty, K. A. Z. Gogins, Y. Mazur, L. L. Miller, J. Org. Chem. 1981, 46, 4545–4551;
- 11cT. Takeya, T. Okubo, S. Nishida, S. Tobinaga, Chem. Pharm. Bull. 1985, 33, 3599–3607;
- 11dH. Nishino, Bull. Chem. Soc. Jpn. 1986, 59, 1733–1739;
- 11eK. Tsunoda, M. Yamane, H. Nishino, K. Kurosawa, Bull. Chem. Soc. Jpn. 1991, 64, 851–856;
- 11fH. Nishino, N. Itoh, M. Nagashima, K. Kurosawa, Bull. Chem. Soc. Jpn. 1992, 65, 620–622;
- 11gD. Planchenault, R. Dhal, J.-P. Robin, Tetrahedron 1993, 49, 5823–5830;
- 11hD. Planchenault, R. Dhal, Tetrahedron 1995, 51, 1395–1404;
- 11iH. Nishino, H. Satoh, M. Yamashita, K. Kurosawa, J. Chem. Soc. Perkin Trans. 2 1999, 1919–1924;
- 11jB. Kramer, A. Averhoff, S. R. Waldvogel, Angew. Chem. 2002, 114, 3013–3014;
Angew. Chem. Int. Ed. 2002, 41, 2981–2982;
10.1002/1521-3773(20020816)41:16<2981::AID-ANIE2981>3.0.CO;2-8 CAS PubMed Web of Science® Google Scholar
- 11kS. R. Waldvogel, Synlett 2002, 622–624.
- 12
- 12aH. F. Hodson, B. Robinson, G. F. Smith, Proc. Chem. Soc. 1961, 465–466;
- 12bE. S. Hall, F. McCapra, A. I. Scott, Tetrahedron 1967, 23, 4131–4141;
- 12cT. Tokuyama, J. W. Daly, Tetrahedron 1983, 39, 41–47;
- 12dY. Adjibade, B. Weniger, J. C. Quirion, B. Kuballa, P. Cabalion, R. Anton, Phytochemistry 1992, 31, 317–319;
- 12eN. H. Lajis, Z. Mahmud, R. F. Toia, Planta Med. 1993, 59, 383–384;
- 12fR. K. Duke, R. D. Allan, G. A. R. Johnston, K. N. Mewett, A. D. Mitrovic, C. C. Duke, T. W. Hambley, J. Nat. Prod. 1995, 58, 1200–1208;
- 12gL. Verotta, T. Pilati, M. Tato, E. Elisabetsky, T. A. Amador, D. S. Nunes, J. Nat. Prod. 1998, 61, 392–396;
- 12hL. Verotta, F. Orsini, M. Sbacchi, M. A. Scheildler, T. A. Amador, E. Elisabetsky, Bioorg. Med. Chem. 2002, 10, 2133–2135.
- 13H. Ishikawa, H. Takayama, N. Aimi, Tetrahedron Lett. 2002, 43, 5637–5639.
- 14
- 14aM. Kunishima, C. Kawachi, F. Iwasaki, K. Terao, S. Tani, Tetrahedron Lett. 1999, 40, 5327–5330;
- 14bM. Kunishima, C. Kawachi, J. Morita, K. Terao, F. Iwasaki, S. Tani, Tetrahedron 1999, 55, 13159–13170;
- 14cM. Kunishima, J. Morita, C. Kawachi, F. Iwasaki, K. Terao, S. Tani, Synlett 1999, 1255–1256;
- 14dM. Kunishima, C. Kawauchi, K. Hioki, K. Terao, S. Tani, Tetrahedron 2001, 57, 1551–1558;
- 14eM. Kunishima, A. Kitao, C. Kawachi, Y. Watanabe, S. Iguchi, K. Hioki, S. Tani, Chem. Pharm. Bull. 2002, 50, 549–550;
- 14fM. Kunishima, T. Ujigawa, Y. Nagaoka, C. Kawachi, K. Hioki, M. Shiro, Chem. Eur. J. 2012, 18, 15856–15867.