Palladium(II)-Catalyzed Dehydrogenative Cross-Coupling between Two C H Bonds: Unexpected CC Bond Formation†
H Bonds: Unexpected CC Bond Formation†
Gaocan Li
Key Laboratory of Green Chemistry and Technology of Ministry of Education, College of Chemistry, and State Key Laboratory of Biotherapy, West China Medical School, Sichuan University, 29 Wangjiang Road, Chengdu 610064 (PR China)
Search for more papers by this authorShengyou Qian
Key Laboratory of Green Chemistry and Technology of Ministry of Education, College of Chemistry, and State Key Laboratory of Biotherapy, West China Medical School, Sichuan University, 29 Wangjiang Road, Chengdu 610064 (PR China)
Search for more papers by this authorChunxia Wang
Key Laboratory of Green Chemistry and Technology of Ministry of Education, College of Chemistry, and State Key Laboratory of Biotherapy, West China Medical School, Sichuan University, 29 Wangjiang Road, Chengdu 610064 (PR China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Jingsong You
Key Laboratory of Green Chemistry and Technology of Ministry of Education, College of Chemistry, and State Key Laboratory of Biotherapy, West China Medical School, Sichuan University, 29 Wangjiang Road, Chengdu 610064 (PR China)
Key Laboratory of Green Chemistry and Technology of Ministry of Education, College of Chemistry, and State Key Laboratory of Biotherapy, West China Medical School, Sichuan University, 29 Wangjiang Road, Chengdu 610064 (PR China)Search for more papers by this authorGaocan Li
Key Laboratory of Green Chemistry and Technology of Ministry of Education, College of Chemistry, and State Key Laboratory of Biotherapy, West China Medical School, Sichuan University, 29 Wangjiang Road, Chengdu 610064 (PR China)
Search for more papers by this authorShengyou Qian
Key Laboratory of Green Chemistry and Technology of Ministry of Education, College of Chemistry, and State Key Laboratory of Biotherapy, West China Medical School, Sichuan University, 29 Wangjiang Road, Chengdu 610064 (PR China)
Search for more papers by this authorChunxia Wang
Key Laboratory of Green Chemistry and Technology of Ministry of Education, College of Chemistry, and State Key Laboratory of Biotherapy, West China Medical School, Sichuan University, 29 Wangjiang Road, Chengdu 610064 (PR China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Jingsong You
Key Laboratory of Green Chemistry and Technology of Ministry of Education, College of Chemistry, and State Key Laboratory of Biotherapy, West China Medical School, Sichuan University, 29 Wangjiang Road, Chengdu 610064 (PR China)
Key Laboratory of Green Chemistry and Technology of Ministry of Education, College of Chemistry, and State Key Laboratory of Biotherapy, West China Medical School, Sichuan University, 29 Wangjiang Road, Chengdu 610064 (PR China)Search for more papers by this authorThis work was supported by grants from the National Basic Research Program of China (973 Program, 2011CB808600), and the National NSF of China (Nos 21025205, 21272160, and 21021001).
Graphical Abstract
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201303099_sm_miscellaneous_information.pdf8.2 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews, see:
- 1aC. C. C. Johansson, T. J. Colacot, Angew. Chem. 2010, 122, 686–718;
10.1002/ange.200903424 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 676–707;
- 1bF. Bellina, R. Rossi, Chem. Rev. 2010, 110, 1082–1146;
- 1cO. Baudoin, Chem. Soc. Rev. 2011, 40, 4902–4911;
- 1dC.-J. Li, Acc. Chem. Res. 2009, 42, 335–344;
- 1eC. S. Yeung, V. M. Dong, Chem. Rev. 2011, 111, 1215–1292;
- 1fC. Zhang, C. Tang, N. Jiao, Chem. Soc. Rev. 2012, 41, 3464–3484.
- 2For selected examples, see:
- 2aZ. Li, D. S. Bohle, C.-J. Li, Proc. Natl. Acad. Sci. USA 2006, 103, 8928–8933;
- 2bS. Lin, C.-X. Song, G.-X. Cai, W.-H. Wang, Z.-J. Shi, J. Am. Chem. Soc. 2008, 130, 12901–12903;
- 2cC. Guo, J. Song, S.-W. Luo, L.-Z. Gong, Angew. Chem. 2010, 122, 5690–5694; Angew. Chem. Int. Ed. 2010, 49, 5558–5562;
- 2dG. Zhang, Y. Zhang, R. Wang, Angew. Chem. 2011, 123, 10613–10616; Angew. Chem. Int. Ed. 2011, 50, 10429–10432;
- 2eJ. Xie, H. Li, J. Zhou, Y. Cheng, C. Zhu, Angew. Chem. 2012, 124, 1278–1281; Angew. Chem. Int. Ed. 2012, 51, 1252–1255;
- 2fJ. Zhang, B. Tiwari, C. Xing, X. Chen, Y. R. Chi, Angew. Chem. 2012, 124, 3709–3712; Angew. Chem. Int. Ed. 2012, 51, 3649–3652.
- 3For selected reviews, see:
- 3aH. Lin, S. J. Danishefsky, Angew. Chem. 2003, 115, 38–53; Angew. Chem. Int. Ed. 2003, 42, 36–51;
- 3bC. Marti, E. M. Carreira, Eur. J. Org. Chem. 2003, 2209–2219;
- 3cC. V. Galliford, K. A. Scheidt, Angew. Chem. 2007, 119, 8902–8912;
10.1002/ange.200701342 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 8748–8758;
- 3dF. Zhou, Y.-L. Liu, J. Zhou, Adv. Synth. Catal. 2010, 352, 1381–1407.
- 4aN. Lindquistl, W. Fenical, J. Am. Chem. Soc. 1991, 113, 2303–2304;
- 4bJ. Li, A. W. G. Burgett, L. Esser, C. Amezcua, P. G. Harran, Angew. Chem. 2001, 113, 4906–4909;
Angew. Chem. Int. Ed. 2001, 40, 4770–4773;
10.1002/1521-3773(20011217)40:24<4770::AID-ANIE4770>3.0.CO;2-T CAS PubMed Web of Science® Google Scholar
- 4cS. M. Verbitski, C. L. Mayne, R. A. Davis, G. P. Concepcion, C. M. Ireland, J. Org. Chem. 2002, 67, 7124–7126;
- 4dJ. Ma, S. M. Hecht, Chem. Commun. 2004, 1190–1191.
- 5
- 5aL. S. Melvin, Jr., L. Conn, US 4686244, August 11, 1987;
- 5bS. Berg, R. Bhat, P. Edwards, S. Hellberg, US 2005/0222181A1, October 6, 2005;
- 5cK.-C. Luk, S.-S. So, J. Zhang, Z. Zhang, WO 2006/136606A2, December 28, 2006;
- 5dB. S. Jensen, CNS Drug Rev. 2002, 8, 353–360;
- 5eT. Jiang, K. L. Kuhen, K. Wolff, H. Yin, K. Bieza, J. Caldwell, B. Bursulaya, T. Tuntland, K. Zhang, D. Karanewsky, Y. He, Bioorg. Med. Chem. Lett. 2006, 16, 2109–2112;
- 5fM. Ochi, K. Kawasaki, H. Kataoka, Y. Uchio, H. Nishi, Biochem. Biophys. Res. Commun. 2001, 283, 1118–1123;
- 5gK. Bernard, S. Bogliolo, J. Ehrenfeld, Br. J. Pharmacol. 2005, 144, 1037–1050.
- 6
- 6aC. M. Passreiter, H. Weber, D. Bläser, R. Boese, Tetrahedron 2002, 58, 279–282;
- 6bB. Sontag, M. Rüth, P. Spiteller, N. Arnold, W. Steglich, M. Reichert, G. Bringmann, Eur. J. Org. Chem. 2006, 1023–1033;
- 6cH. M. Ge, C. H. Zhu, D. H. Shi, L. D. Zhang, D. Q. Xie, J. Yang, S. W. Ng, R. X. Tan, Chem. Eur. J. 2008, 14, 376–381;
- 6dA. Closse, W. Haefliger, D. Hauser, US 4252817, February 24, 1981;
- 6eS. B. Kadin, J. Med. Chem. 1972, 15, 551–552;
- 6fA. Closse, W. Haefliger, D. Hauser, J. Med. Chem. 1981, 24, 1465–1471;
- 6gM. Lardic, C. Party, M. Duflos, J. Guillon, S. Massip, F. Cruzalegui, T, Edmonds, S. Giraudet, L. Marini, S. Leonce, J. Enzym. Inhib. Chem. 2006, 21, 313–325;
- 6hY.-J. Kwon, M.-J. Sohn, C.-J. Zheng, W.-G. Kim, Org. Lett. 2007, 9, 2449–2451.
- 7
- 7aR. Sarges, H. R. Howard, B. K. Koe, A. Weissman, J. Med. Chem. 1989, 32, 437–444;
- 7bM. S. C. Pedras, J. L. Sorensen, F. I. Okanga, I. L. Zaharia, Bioorg. Med. Chem. Lett. 1999, 9, 3015–3020;
- 7cA. Andreani, S. Bellini, S. Burnelli, M. Granaiola, A. Leoni, A. Locatelli, R. Morigi, M. Rambaldi, L. Varoli, N. Calonghi, C. Cappadone, M. Zini, C. Stefanelli, L. Masotti, R. H. Shoemaker, J. Med. Chem. 2010, 53, 5567–5575;
- 7dM. Mohammadi, G. McMahon, L. Sun, C. Tang, P. Hirth, B. K. Yeh, S. R. Hubbard, J. Schlessinger, Science 1997, 276, 955–960;
- 7eJ. C. Henise, J. Taunton, J. Med. Chem. 2011, 54, 4133–4146;
- 7fG.-B. Deng, Z.-Q. Wang, R.-J. Song, M.-B. Zhou, W.-T. Wei, P. Xie, J.-H. Li, Chem. Commun. 2011, 47, 8151–8153.
- 8For selected examples, see:
- 8aR. A. Altman, A. M. Hyde, X. Huang, S. L. Buchwald, J. Am. Chem. Soc. 2008, 130, 9613–9620;
- 8bA. M. Taylor, R. A. Altman, S. L. Buchwald, J. Am. Chem. Soc. 2009, 131, 9900–9901;
- 8cB. M. Trost, J. Xie, J. D. Sieber, J. Am. Chem. Soc. 2011, 133, 20611–20622;
- 8dY.-Y. Han, Z.-J. Wu, W.-B. Chen, X.-L. Du, X.-M. Zhang, W.-C. Yuan, Org. Lett. 2011, 13, 5064–5067.
- 9
- 9aA. M. Berman, J. S. Johnson, J. Am. Chem. Soc. 2004, 126, 5680–5681;
- 9bE. J. Yoo, S. Ma, T.-S. Mei, K. S. L. Chan, J.-Q. Yu, J. Am. Chem. Soc. 2011, 133, 7652–7655;
- 9cN. Matsuda, K. Hirano, T. Satoh, M. Miura, Angew. Chem. 2012, 124, 3702–3705; Angew. Chem. Int. Ed. 2012, 51, 3642–3645;
- 9dA. M. Dumas, G. A. Molander, J. W. Bode, Angew. Chem. 2012, 124, 5781–5784;
10.1002/ange.201201077 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 5683–5686;
- 9eR. P. Rucker, A. M. Whittaker, H. Dang, G. Lalic, J. Am. Chem. Soc. 2012, 134, 6571–6574;
- 9fN. Matsuda, K. Hirano, T. Satoh, M. Miura, Angew. Chem. 2012, 124, 11997–12001; Angew. Chem. Int. Ed. 2012, 51, 11827–11831;
- 9gY. Miki, K. Hirano, T. Satoh, M. Miura, Org. Lett. 2013, 15, 172–175.
- 10CCDC 933727 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 11S. P. Wathen, A. W. Czarnik, J. Org. Chem. 1992, 57, 6129–6133.
- 12The O-benzoyl hydroxylamines of acyclic amines were inactive in this coupling reaction, probably due to the instability of the acyclic imine intermediate in the current catalytic system.
- 13E. Boess, C. Schmitz, M. Klussmann, J. Am. Chem. Soc. 2012, 134, 5317–5325.
- 14
- 14aM. R. Monaco, P. Renzi, D. M. S. Schietroma, M. Bella, Org. Lett. 2011, 13, 4546–4549;
- 14bG. P. Claxton, L. Allen, J. M. Grisar, Org. Synth. 1977, 56, 118–121;
- 14cG. P. Claxton, L. Allen, J. M. Grisar, Org. Synth. 1988, Coll. Vol. 6, 968–971.



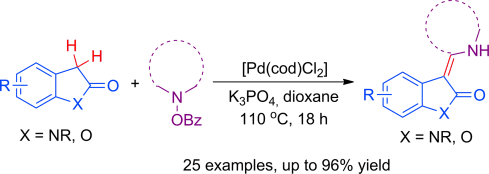
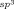 bonds through reactions of indolin-2-ones or benzofuran-2-ones with O-benzoyl hydroxylamines in the absence of an added oxidant.
bonds through reactions of indolin-2-ones or benzofuran-2-ones with O-benzoyl hydroxylamines in the absence of an added oxidant.