An Anion-Modulated Three-Way Supramolecular Switch that Selectively Binds Dihydrogen Phosphate, H2PO4−†
Jesse V. Gavette
Department of Chemistry and Biochemistry & Materials Science Institute, University of Oregon, Eugene, OR 97403-1253 (USA)
Search for more papers by this authorProf. Dr. Nancy S. Mills
Department of Chemistry, Trinity University, San Antonio, TX 78212 (USA)
Search for more papers by this authorDr. Lev N. Zakharov
CAMCOR—Center for Advanced Materials Characterization in Oregon, University of Oregon, Eugene, OR 97403-1443 (USA)
Search for more papers by this authorDr. Charles A. Johnson II
Department of Chemistry and Biochemistry & Materials Science Institute, University of Oregon, Eugene, OR 97403-1253 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Darren W. Johnson
Department of Chemistry and Biochemistry & Materials Science Institute, University of Oregon, Eugene, OR 97403-1253 (USA)
Department of Chemistry and Biochemistry & Materials Science Institute, University of Oregon, Eugene, OR 97403-1253 (USA)Search for more papers by this authorCorresponding Author
Prof. Dr. Michael M. Haley
Department of Chemistry and Biochemistry & Materials Science Institute, University of Oregon, Eugene, OR 97403-1253 (USA)
Department of Chemistry and Biochemistry & Materials Science Institute, University of Oregon, Eugene, OR 97403-1253 (USA)Search for more papers by this authorJesse V. Gavette
Department of Chemistry and Biochemistry & Materials Science Institute, University of Oregon, Eugene, OR 97403-1253 (USA)
Search for more papers by this authorProf. Dr. Nancy S. Mills
Department of Chemistry, Trinity University, San Antonio, TX 78212 (USA)
Search for more papers by this authorDr. Lev N. Zakharov
CAMCOR—Center for Advanced Materials Characterization in Oregon, University of Oregon, Eugene, OR 97403-1443 (USA)
Search for more papers by this authorDr. Charles A. Johnson II
Department of Chemistry and Biochemistry & Materials Science Institute, University of Oregon, Eugene, OR 97403-1253 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Darren W. Johnson
Department of Chemistry and Biochemistry & Materials Science Institute, University of Oregon, Eugene, OR 97403-1253 (USA)
Department of Chemistry and Biochemistry & Materials Science Institute, University of Oregon, Eugene, OR 97403-1253 (USA)Search for more papers by this authorCorresponding Author
Prof. Dr. Michael M. Haley
Department of Chemistry and Biochemistry & Materials Science Institute, University of Oregon, Eugene, OR 97403-1253 (USA)
Department of Chemistry and Biochemistry & Materials Science Institute, University of Oregon, Eugene, OR 97403-1253 (USA)Search for more papers by this authorThis work was supported by NIH grant R01-GM087398, which also funded early stage intellectual property that was licensed by SupraSensor Technologies, a company co-founded by the principal investigators. We appreciate extremely helpful conversations with Prof. Pablo Ballester at ICIQ, Spain in revising some of the complex solution speciation presented herein. University of Oregon NMR facilities are supported by a grant from the National Science Foundation (CHE-0923589).
Graphical Abstract
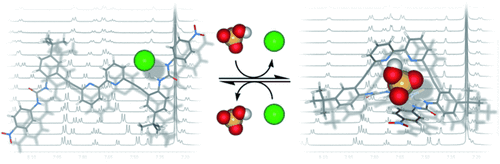
An anionic three-way switch: A bipyridyl bis(urea)-based anion receptor that is highly selective for dihydrogen phosphate demonstrates spectroscopically distinct anion-bound conformations toward halides and select oxoanions. 1H NMR studies show the differing anion-induced conformations are reversible allowing this system to function as a three-way molecular switch.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201302929_sm_miscellaneous_information.pdf4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. Zhang, K. Zhu, F. Huang, in Supramolecular Chemistry From Molecules to Nanomaterials, Wiley, Chichester, 2012, pp. 2425–2496;
- 1bV. Balzani, A. Credi, F. M. Raymo, J. F. Stoddart, Angew. Chem. 2000, 112, 3484–3530;
10.1002/1521-3757(20001002)112:19<3484::AID-ANGE3484>3.0.CO;2-O Google ScholarAngew. Chem. Int. Ed. 2000, 39, 3348–3391;10.1002/1521-3773(20001002)39:19<3348::AID-ANIE3348>3.0.CO;2-X CAS PubMed Web of Science® Google Scholar
- 1cE. R. Kay, D. A. Leigh, F. Zerbetto, Angew. Chem. 2007, 119, 72–196; Angew. Chem. Int. Ed. 2007, 46, 72–191.
- 2K. C.-F. Leung, C.-P. Chak, C.-M. Lo, W.-Y. Wong, S. Xuan, C. H. K. Cheng, Chem. Asian J. 2009, 4, 364–381.
- 3
- 3aI. M. Jones, A. D. Hamilton, Angew. Chem. 2011, 123, 4693–4696; Angew. Chem. Int. Ed. 2011, 50, 4597–4600;
- 3bK. Sato, Y. Nishina, K. Shiga, J. Biochem. 1992, 111, 359–365;
- 3cL. Guo, Q.-L. Wang, Q.-Q. Jiang, Q.-J. Jiang, Y.-B. Jiang, J. Org. Chem. 2007, 72, 9947–9953;
- 3dY.-L. Huang, W.-C. Hung, C.-C. Lai, Y.-H. Liu, S.-M. Peng, S.-H. Chiu, Angew. Chem. 2007, 119, 6749–6753; Angew. Chem. Int. Ed. 2007, 46, 6629–6633;
- 3eC.-F. Lin, C.-C. Lai, Y.-H. Liu, S.-M. Peng, S.-H. Chiu, Chem. Eur. J. 2007, 13, 4350–4355;
- 3fM. J. Chmielewski, J. Jurczak, Chem. Eur. J. 2006, 12, 7652–7667;
- 3gC. J. Serpell, R. Chall, A. L. Thompson, P. D. Beer, Dalton Trans. 2011, 40, 12052–12055;
- 3hT.-C. Lin, C.-C. Lai, S.-H. Chiu, Org. Lett. 2009, 11, 613–616;
- 3iD. Makuc, J. R. Hiscock, M. E. Light, P. A. Gale, J. Plavec, Beilstein J. Org. Chem. 2011, 7, 1205–1214;
- 3jJ. Suk, V. R. Naidu, X. Liu, M. S. Lah, K.-S. Jeong, J. Am. Chem. Soc. 2011, 133, 13938–13941;
- 3kK.-S. Jeong, M. K. Chae, J. Suk, Pure Appl. Chem. 2012, 84, 953–964.
- 4
- 4aA. N. Swinburne, M. J. Paterson, A. Beeby, J. W. Steed, Chem. Eur. J. 2010, 16, 2714–2718;
- 4bM. J. Kim, H.-W. Lee, D. Moon, K.-S. Jeong, Org. Lett. 2012, 14, 5018–5021;
- 4cM. H. Filby, S. J. Dickson, N. Zaccheroni, L. Prodi, S. Bonacchi, M. Montalti, M. J. Paterson, T. D. Humphries, C. Chiorboli, J. W. Steed, J. Am. Chem. Soc. 2008, 130, 4105–4113;
- 4dT. Pierro, C. Gaeta, F. Troisi, P. Neri, Tetrahedron Lett. 2009, 50, 350–353;
- 4eK. J. Wallace, W. J. Belcher, D. R. Turner, K. F. Syed, J. W. Steed, J. Am. Chem. Soc. 2003, 125, 9699–9715;
- 4fS. Moerkerke, M. Ménand, I. Jabin, Chem. Eur. J. 2010, 16, 11712–11719.
- 5D. E. Koshland, Angew. Chem. 1994, 106, 2468–2472; Angew. Chem. Int. Ed. Engl. 1994, 33, 2375–2378.
- 6For a recent review on the importance and examples of coordination of phosphate and phosphorylated guest by supramolecular receptors, see: A. E. Hargrove, S. Nieto, T. Zhang, J. L. Sessler, E. V. Anslyn, Chem. Rev. 2011, 111, 6603–6782.
- 7
- 7aP. A. Gale, J. R. Hiscock, S. J. Moore, C. Caltagirone, M. B. Hursthouse, M. E. Light, Chem. Asian J. 2010, 5, 555–561;
- 7bP. A. Gale, J. R. Hiscock, N. Lalaoui, M. E. Light, N. J. Wells, M. Wenzel, Org. Biomol. Chem. 2012, 10, 5909–5915;
- 7cS. Kondo, Y. Hiraoka, N. Kurumatani, Y. Yano, Chem. Commun. 2005, 1720–1722;
- 7dM. A. Saeed, D. R. Powell, M. A. Hossain, Tetrahedron Lett. 2010, 51, 4904–4907;
- 7eW. Gong, S. Bao, F. Wang, J. Ye, G. Ning, K. Hiratani, Tetrahedron Lett. 2011, 52, 630–634;
- 7fT. H. Kwon, K.-S. Jeong, Tetrahedron Lett. 2006, 47, 8539–8541;
- 7gS. Kondo, R. Takai, Org. Lett. 2013, 15, 538–541.
- 8
- 8aO. B. Berryman, C. A. Johnson, L. N. Zakharov, M. M. Haley, D. W. Johnson, Angew. Chem. 2008, 120, 123–126;
10.1002/ange.200703971 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 117–120;
- 8bC. A. Johnson, O. B. Berryman, A. C. Sather, L. N. Zakharov, M. M. Haley, D. W. Johnson, Cryst. Growth Des. 2009, 9, 4247–4249;
- 8cC. N. Carroll, O. B. Berryman, C. A. Johnson, L. N. Zakharov, M. M. Haley, D. W. Johnson, Chem. Commun. 2009, 2520–2522;
- 8dC. N. Carroll, B. A. Coombs, S. P. McClintock, C. A. Johnson II, O. B. Berryman, D. W. Johnson, M. M. Haley, Chem. Commun. 2011, 47, 5539–5541.
- 9
- 9aE. Weber, H. P. Josel, H. Puff, S. Franken, J. Org. Chem. 1985, 50, 3125–3132;
- 9bC. M. Amb, S. C. Rasmussen, J. Org. Chem. 2006, 71, 4696–4699.
- 10W. B. Wan, M. M. Haley, J. Org. Chem. 2001, 66, 3893–3901.
- 11
- 11aP. Gans, A. Sabatini, A. Vacca, Talanta 1996, 43, 1739–1753;
- 11bC. Frassineti, S. Ghelli, P. Gans, A. Sabatini, M. S. Moruzzi, A. Vacca, Anal. Biochem. 1995, 231, 374–382.
- 12S. Lee, A. H. Flood, Top. Heterocycl. Chem. 2012, 28, 85–108.
- 13
- 13aGaussian 09, Revision C.01, M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, et al., Gausian, Inc., Wallingford CT, 2010;
- 13bJ.-D. Chai, M. Head-Gordon, Phys. Chem. Chem. Phys. 2008, 10, 6615–6615;
- 13cM. Swart, M. Sola, F. M. Bickelhaupt, J. Chem. Phys. 2009, 131, 094103.
- 14
- 14aI. C. Calder, T. M. Spotswood, C. I. Tanzer, Aust. J. Chem. 1967, 20, 1195–1212;
- 14bT. M. Spotswood, C. I. Tanzer, Aust. J. Chem. 1967, 20, 1213–1225;
- 14cT. M. Spotswood, C. I. Tanzer, Aust. J. Chem. 1967, 20, 1227–1242.
- 15A combination of the high symmetry of the receptor, borderline molecular weight for NOESY and ROESY experiments, significant overlap of aryl peaks of the strongest complex (1⋅H2PO4−), and weak binding in all of the other systems prevented any conclusive conformational assignment of anion complexes in solution by these methods.
- 16X-ray data for 1⋅(TBABr)2: C80H114Br2N10O6, Mr=1471.63, crystal size 0.34×0.10×0.01 mm3, Triclinic, space group P
 , a=8.496(8), b=12.527(12), c=19.598(18) Å, α=100.299(16), β=93.450(15), γ=92.059(14)°, V=2046(3) Å3, Z=1, Z′=0.5, ρcalcd=1.194 g cm−3, μ=1.045 mm−1, F(000)=782, Mo Kα radiation, λ=0.71073 Å, T=173(2) K, 2Θmax=45.00°, 11 442 reflections measured [Rint=0.1774], 5324 reflections observed, 443 refined parameters, R1=0.0926, wR2=0.1626, and GOF=1.003 for reflections with I>2σ(I), R1=0.2375, wR2=0.2146, and GOF=1.003 for all data, max/min residual electron density +0.490/−0.714 e Å−3. CCDC 930545 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
, a=8.496(8), b=12.527(12), c=19.598(18) Å, α=100.299(16), β=93.450(15), γ=92.059(14)°, V=2046(3) Å3, Z=1, Z′=0.5, ρcalcd=1.194 g cm−3, μ=1.045 mm−1, F(000)=782, Mo Kα radiation, λ=0.71073 Å, T=173(2) K, 2Θmax=45.00°, 11 442 reflections measured [Rint=0.1774], 5324 reflections observed, 443 refined parameters, R1=0.0926, wR2=0.1626, and GOF=1.003 for reflections with I>2σ(I), R1=0.2375, wR2=0.2146, and GOF=1.003 for all data, max/min residual electron density +0.490/−0.714 e Å−3. CCDC 930545 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.