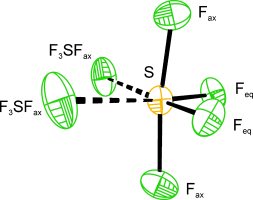
The Solid-State Structure of SF4: The Final Piece of the Puzzle†
This work was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC).
Graphical Abstract
Solved at last: The crystal structure of solid SF4, which has a melting point of −121 °C, has been obtained. It exhibits weak intermolecular S⋅⋅⋅F interactions. A similar structural motif was observed within a layer of SF4 in [HNC5H3(CH3)2+]2F−⋅⋅⋅SF4[SF5−]⋅3 SF4. The latter structure contains a range of bonding modes between S and F, namely SF5−, F4S⋅⋅⋅F−, F4S⋅⋅⋅FSF4−, and F4S⋅⋅⋅FSF3.