SiSi and SiO Bond Activation at Platinum: Stepwise Formation of a SiH3 Complex†
Dipl.-Chem. Cathérine Mitzenheim
Humboldt-Universität zu Berlin, Department of Chemistry, Brook-Taylor-Strasse 2, 12489 Berlin (Gemany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Thomas Braun
Humboldt-Universität zu Berlin, Department of Chemistry, Brook-Taylor-Strasse 2, 12489 Berlin (Gemany)
Humboldt-Universität zu Berlin, Department of Chemistry, Brook-Taylor-Strasse 2, 12489 Berlin (Gemany)Search for more papers by this authorDipl.-Chem. Cathérine Mitzenheim
Humboldt-Universität zu Berlin, Department of Chemistry, Brook-Taylor-Strasse 2, 12489 Berlin (Gemany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Thomas Braun
Humboldt-Universität zu Berlin, Department of Chemistry, Brook-Taylor-Strasse 2, 12489 Berlin (Gemany)
Humboldt-Universität zu Berlin, Department of Chemistry, Brook-Taylor-Strasse 2, 12489 Berlin (Gemany)Search for more papers by this authorWe would like to acknowledge the Deutsche Forschungsgesellschaft for financial support. We are grateful to Dr. S. Mebs for the crystallography.
Graphical Abstract
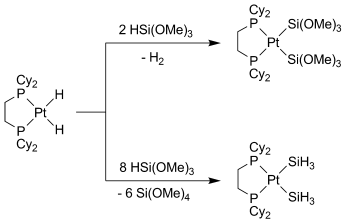
The more, the better: Treatment of [Pt(H)2(dcpe)] (dcpe=1,2-bis(dicyclohexylphosphino)ethane) with stoichiometric amounts of silane leads to the bis(silyl) complex [Pt{Si(OMe)3}2(dcpe)], whereas the use of an excess silane results in hydrodealkoxylations by repetitive SiO bond activation and the formation of SiH3 ligands (see scheme; Cy=cyclohexyl).
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201302459_sm_miscellaneous_information.pdf210 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. Y. Corey, J. Braddock-Wilking, Chem. Rev. 1999, 99, 175–292;
- 1bJ. Y. Corey, Chem. Rev. 2011, 111, 863–1071.
- 2H. K. Sharma, K. H. Pannell, Chem. Rev. 1995, 95, 1351–1374.
- 3J. Y. Corey, Adv. Organomet. Chem. 2004, 51, 1–52.
- 4A. Roscher, A. Bockholt, T. Braun, Dalton Trans. 2009, 1378–1382.
- 5M. J. Michalczyk, C. A. Recatto, J. C. Calabrese, M. J. Fink, J. Am. Chem. Soc. 1992, 114, 7955–7957.
- 6
- 6aL. Hao, A.-M. Lebuis, J. F. Harrod, E. Samuel, Chem. Commun. 1997, 2193–2194;
- 6bL. Hao, A.-M. Lebuis, J. F. Harrod, Chem. Commun. 1998, 1089–1090;
- 6cJ. F. Harrod, Coord. Chem. Rev. 2000, 206–207, 493–531.
- 7H.-G. Woo, R. H. Heyn, T. D. Tilley, J. Am. Chem. Soc. 1992, 114, 5698–5707.
- 8
- 8aB. J. Aylett, J. M. Campbell, J. Chem. Soc. A 1969, 1910–1916;
- 8bB. J. Aylett, J. M. Campbell, A. Walton, J. Chem. Soc. A 1969, 2110–2113;
- 8cA. P. Hagen, C. R. Higgins, P. J. Russo, Inorg. Chem. 1971, 10, 1657–1660;
- 8dJ. S. Allinson, B. J. Aylett, H. M. Colquhoun, J. Organomet. Chem. 1976, 112, C 7–C8.
- 9
- 9aS. Schmitzer, U. Weis, H. Kab, W. Buchner, W. Malisch, T. Polzer, U. Posset, W. Kiefer, Inorg. Chem. 1993, 32, 303–309;
- 9bW. Malisch, S. Moller, O. Fey, H.-U. Wekel, R. Pikl, U. Posset, W. Kiefer, J. Organomet. Chem. 1996, 507, 117–124.
- 10
- 10aI. Atheaux, B. Donnadieu, V. Rodriguez, S. Sabo-Etienne, B. Chaudret, K. Hussein, J.-C. Barthelat, J. Am. Chem. Soc. 2000, 122, 5664–5665;
- 10bR. B. Said, K. Hussein, J.-C. Barthelat, I. Atheaux, S. Sabo-Etienne, M. Grellier, B. Donnadieu, B. Chaudret, Dalton Trans. 2003, 4139–4146;
- 10csee also: M. C. Lipke, T. D. Tilley, Angew. Chem. 2012, 124, 11277–11283; Angew. Chem. Int. Ed. 2012, 51, 11115–11121.
- 11J.-P. Djukic, F. Rose-Munch, E. Rose, F. Simon, Organometallics 1995, 14, 2027–2038.
- 12Note that 1 was generated in situ by reaction of [Pt(Cl)2(dcpe)] with lithium triethylborohydride:
- 12aC. A. Jaska, A. J. Lough, I. Manners, Dalton Trans. 2005, 326–331;
- 12bD. J. Schwartz, R. A. Andersen, J. Am. Chem. Soc. 1995, 117, 4014–4025.
- 13B. J. Coe, S. J. Glenwright, Coord. Chem. Rev. 2000, 203, 5–80.
- 14Union Carbide Co., US Pat., 2 606 811, 1952; Dow Corning Co., US Pat., 3 639 105, 1972; Dow Corning Co., US Pat., 6 013 235, 2000.
- 15
- 15aJ. Voigt, M. A. Chilleck, T. Braun, Dalton Trans. 2013, 42, 4052–4058;
- 15bfor a catalytic reaction at rhodium see: L. Rosenberg, C. W. Davis, J. Yao, J. Am. Chem. Soc. 2001, 123, 5120–5121.
- 16J. Voigt, T. Braun, Dalton Trans. 2011, 40, 12699–12704.
- 17J. Pfeiffer, U. Schubert, Organometallics 1999, 18, 3245–3248.
- 18D. Chan, S. B. Duckett, S. L. Heath, I. G. Khazal, R. N. Perutz, S. Sabo-Etienne, P. L. Timmins, Organometallics 2004, 23, 5744–5756.
- 19E. A. V. Ebsworth, V. M. Marganian, F. J. S. Reed, R. O. Gould, J. Chem. Soc. Dalton Trans. 1978, 1167–1170.
- 20X-ray structure analysis of 4: C32H60P2PtSi2, Mr=758.01, crystal dimensions 0.29×0.28×0.27 mm3; monoclinic; P21/c; a=12.1816(2), b=17.9500(3), c=16.6358(3) Å, β=101.302(2)°, Z=4, V=3567.04(11) Å3, ρcalcd=1.411 mg m−3; 2θmax=28.45°, MoKα radiation (λ=0.71073), T=100(2) K, 57814 reflections collected, 8975 were unique (Rint=0.0694); STOE IPDS 2Θ diffractometer; multiscan absorption correction (min./max transmission 0.3587/0.4424), μ=4.109 mm−1. The structure was solved by direct methods and was refined with full-matrix least-squares methods on F 2 (SHELX-97).[27] Final R1, wR2 values on all data: 0.0277, 0.0351; R1, wR2 values for 8975 reflections with I0>2σ(I0): 0.0171, 0.0343; residual electron density +0.654/−0.986 e Å3. Hydrogen atoms were placed at calculated positions and refined using a riding model. The hydrogen atoms on the silicon were located on the difference Fourier map and refined isotropically. CCDC 930069 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 21J. Braddock-Wilking, Y. Levchinsky, N. P. Rath, Organometallics 2000, 19, 5500–5510.
- 22D. C. Pestana, T. S. Koloski, D. H. Berry, Organometallics 1994, 13, 4173–4175.
- 23G. P. Mitchell, T. D. Tilley, J. Am. Chem. Soc. 1998, 120, 7635–7636.
- 24
- 24aH. Tobita, K. Ueno, M. Shimoi, H. Ogino, J. Am. Chem. Soc. 1990, 112, 3415–3420;
- 24bT. Takeuchi, H. Tobita, H. Ogino, Organometallics 1991, 10, 835–836;
- 24cH. Ogino, Chem. Rec. 2002, 2, 291–306;
- 24dM. Besora, F. Maseras, A. Lledós, O. Eisenstein, Inorg. Chem. 2002, 41, 7105–7112;
- 24eS. Park, B. G. Kim, I. Göttker-Schnetmann, M. Brookhart, ACS Catal. 2012, 2, 307–316.
- 25M. Hackett, G. M. Whitesides, J. Am. Chem. Soc. 1988, 110, 1449–1462.
- 26
- 26aK. Thorshaug, O. Swang, I. M. Dahl, A. Olafsen, J. Phys. Chem. A 2006, 110, 9801–9804;
- 26bE. A. V. Ebsworth, J. J. Turner, J. Chem. Phys. 1962, 36, 2628–2634.
- 27aSHELX-97, G. M. Sheldrick, program for crystal structure refinement, University of Göttingen, 1997;
- 27bG. M. Sheldrick, Acta Crystallogr. Sect. A 2008, 64, 112–122.