Chemoselective Oxidation by Electronically Tuned Nitroxyl Radical Catalysts†
Shohei Hamada
Institute for Chemical Research, Kyoto University, Uji, Kyoto 611-0011(Japan)
Search for more papers by this authorDr. Takumi Furuta
Institute for Chemical Research, Kyoto University, Uji, Kyoto 611-0011(Japan)
Search for more papers by this authorYoshiyuki Wada
Institute for Chemical Research, Kyoto University, Uji, Kyoto 611-0011(Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Takeo Kawabata
Institute for Chemical Research, Kyoto University, Uji, Kyoto 611-0011(Japan)
Institute for Chemical Research, Kyoto University, Uji, Kyoto 611-0011(Japan)Search for more papers by this authorShohei Hamada
Institute for Chemical Research, Kyoto University, Uji, Kyoto 611-0011(Japan)
Search for more papers by this authorDr. Takumi Furuta
Institute for Chemical Research, Kyoto University, Uji, Kyoto 611-0011(Japan)
Search for more papers by this authorYoshiyuki Wada
Institute for Chemical Research, Kyoto University, Uji, Kyoto 611-0011(Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Takeo Kawabata
Institute for Chemical Research, Kyoto University, Uji, Kyoto 611-0011(Japan)
Institute for Chemical Research, Kyoto University, Uji, Kyoto 611-0011(Japan)Search for more papers by this authorWe are grateful to Prof. Norihiro Tokitoh and Dr. Takahiro Sasamori for generous assistance to the measurement of cyclic voltammetry. This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas “Advanced Molecular Transformation by Organocatalysts” from The Ministry of Education, Culture, Sports, Science and Technology (Japan), and by Grant-in-Aid for JSPS Fellows to S.H.
Graphical Abstract
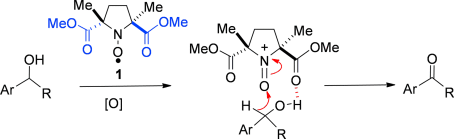
Electronic tuning: Nitroxyl radical 1 is shown to be an efficient catalyst for the oxidation of secondary alcohols, and promotes oxidation through an oxoammonium species which is highly reactive because of the adjacent electron-withdrawing ester groups. Chemoselective oxidation of benzylic alcohols in the presence of aliphatic alcohols is observed and is proposed to proceed by a rate-determining hydride transfer.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201302261_sm_miscellaneous_information.pdf2.3 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews, see:
- 1aA. E. J. de Nooy, A. C. Besemer, H. van Bekkum, Synthesis 1996, 1153–1174;
- 1bR. A. Sheldon, I. W. C. E. Arends, Adv. Synth. Catal. 2004, 346, 1051–1071;
- 1cL. Tebben, A. Studer, Angew. Chem. 2011, 123, 5138–5174;
10.1002/ange.201002547 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 5034–5068.
- 2
- 2aM. F. Semmelhack, C. S. Chou, D. A. Cortes, J. Am. Chem. Soc. 1983, 105, 4492–4494;
- 2bR. Siedlecka, J. Skarzewski, J. Mlochowski, Tetrahedron Lett. 1990, 31, 2177–2180;
- 2cN. J. Davis, S. L. Flitsch, Tetrahedron Lett. 1993, 34, 1181–1184;
- 2dA. E. J. de Nooy, A. C. Basemer, H. van Bekkum, Tetrahedron 1995, 51, 8023–8032;
- 2eA. E. J. de Nooy, A. C. Basemer, H. van Bekkum, Carbohydr. Res. 1995, 269, 89–98;
- 2fJ. Einhorn, C. Einhorn, F. Ratajczak, J. L. Pierre, J. Org. Chem. 1996, 61, 7452–7454;
- 2gA. De Mico, R. Margarita, L. Parlanti, A. Vescovi, G. Piancatelli, J. Org. Chem. 1997, 62, 6974–6977;
- 2hL. De Luca, G. Giacomelli, A. Porcheddu, Org. Lett. 2001, 3, 3041–3043;
- 2iJ. M. Hoover, S. S. Stahl, J. Am. Chem. Soc. 2011, 133, 16901–16910.
- 3
- 3aV. A. Golubev, V. N. Borislavskii, A. L. Aleksandrov, Izv. Akad. Nauk USSR Ser. Khim. 1977, 9, 2025–2034;
- 3bM. F. Semmelhack, C. R. Schmid, D. A. Cortés, Tetrahedron Lett. 1986, 27, 1119–1122;
- 3cZ. Ma, J. M. Bobbitt, J. Org. Chem. 1991, 56, 6110–6114.
- 4
- 4aK. Adamic, D. F. Bowman, T. Gillan, K. U. Ingold, J. Am. Chem. Soc. 1971, 93, 902–908;
- 4bD. F. Bowman, T. Gillan, K. U. Ingold, J. Am. Chem. Soc. 1971, 93, 6555–6561;
- 4cJ. Martinie-Hombrouck, A. Rassat, Tetrahedron 1974, 30, 433–436.
- 5
- 5aM. Shibuya, M. Tomizawa, I. Suzuki, Y. Iwabuchi, J. Am. Chem. Soc. 2006, 128, 8412–8413;
- 5bM. Shibuya, T. Sato, M. Tomizawa, Y. Iwabuchi, Chem. Commun. 2009, 1739–1741;
- 5cM. Shibuya, Y. Osada, Y. Sasano, M. Tomizawa, Y. Iwabuchi, J. Am. Chem. Soc. 2011, 133, 6497–6500.
- 6Y. Demizu, H. Shiigi, T. Oda, Y. Matsumura, O. Onomura, Tetrahedron Lett. 2008, 49, 48–52.
- 7Inokuchi and co-workers developed TEMPO derivatives possessing the OCOR group at the 4-position. These derivatives were expected be more reactive catalysts than TEMPO because of the inductive effects of the remote 4-OCOR groups, and have been effectively used for the oxidation of secondary alcohols in the presence of stoichiometric amount of Py HBr3. The difference in E0 between TEMPO and the 4-OCOCF3 derivative was reported to be 121 mV. See: Z.-W. Mei, T. Omote, M. Mansour, H. Kawafuchi, Y. Takaguchi, A. Jutand, S. Tsuboi, T. Inokuchi, Tetrahedron 2008, 64, 10761–10766.
- 85-F-AZADO was claimed to be a more reactive catalyst than AZADO because of the presence of an electron-withdrawing F atom at the remote position. The difference in E0 between AZADO and the 5-F-AZADO was reported to be 177 mV. See Ref. [5c].
- 9We have proposed a hydrogen-bonding interaction between the catalyst and the substrate as the key to regioselective acylation promoted by functionalized pyrrolidinopyridine catalysts. See:
- 9aT. Kawabata, W. Muramatsu, T. Nishio, T. Shibata, H. Schedel, J. Am. Chem. Soc. 2007, 129, 12890–12895;
- 9bK. Yoshida, K. Mishiro, Y. Ueda, T. Shigeta, T. Furuta, T. Kawabata, Adv. Synth. Catal. 2012, 354, 3291–3298;
- 9cK. Yoshida, T. Shigeta, T. Furuta, T. Kawabata, Chem. Commun. 2012, 48, 6981–6983.
- 10J. Einhorn, C. Einhorn, F. Ratajczak, J- ;L. Pierre, Synth. Commun. 2000, 30, 1837–1848.
- 11The reference electrode was Ag/Ag+. For details, see the Supporting information.
- 12Niroxyl radical catalysts such as TEMPO derivatives possessing OCOR group at the 4-position (Ref. [7]) and 5-F-AZADO (Ref. [8]) have been reported to be more active catalysts compared to the parent TEMPO and AZADO, respectively, because of the electron-withdrawing substituents. Because the electron-withdrawing substituents are located at a position remote from the active site (nitroxyl group), the electronic effects in these catalysts seems to be much smaller than the present case. The extent of the electronic effects may be suggested by the difference in their redox potentials: The differences in E0 between TEMPO and the 4-OCOCF3 derivative (Ref. [7]), AZADO and the 5-F-AZADO (Ref. [8]), and PROXYL and 1 (this work) were 121 mV, 177 mV, and 422 mV, respectively.
- 13
- 13aH. Tohma, S. Takizawa, T. Maegawa, Y. Kita, Angew. Chem. 2000, 112, 1362–1364;
10.1002/(SICI)1521-3757(20000403)112:7<1362::AID-ANGE1362>3.0.CO;2-G Google ScholarAngew. Chem. Int. Ed. 2000, 39, 1306–1308;10.1002/(SICI)1521-3773(20000403)39:7<1306::AID-ANIE1306>3.0.CO;2-J CAS PubMed Web of Science® Google Scholar
- 13bH. Tohma, Y. Kita, Adv. Synth. Catal. 2004, 346, 111–124.
- 14For a recent example of chemoselective oxidation of benzylic alcohol in the presence of aliphatic alcohol, see: H. Ruijun, L. Ming, W. Hegeng, W. Yanguang, Chin. J. Chem. 2009, 27, 587–592.
- 15Vicinal diols have been known to undergo oxidative cleavage in the presence of IIII reagents, see:
- 15aK. C. Nicolaou, V. A. Adsool, C. R. H. Hale, Org. Lett. 2010, 12, 1552–1555;
- 15bM. Shibuya, T. Shibuta, H. Fukuda, Y. Iwabuchi, Org. Lett. 2012, 14, 5010–5013.
- 16Recently, chemoselective oxidation of allylic and benzylic alcohols with an N-hydroxyindole/CuI/air system has been reported: S. Shen, V. Kartika, Y. S. Tan, R. D. Webster, K. Narasaka, Tetrahedron Lett. 2012, 53, 986–990.
- 17The kinetic isotope effect of the oxidation of an aliphatic alcohol, 3-phenylpropanol, with a 1/PIFA system was also measured under pseudo-first-order conditions, and determined to be kH/kD=1.7 (for details see the Supporting Inforamtion).
- 18A hydride transfer mechanism has been proposed in TEMPO-mediated oxidation of alcohols based on computational studies: W. F. Bailey, J. M. Bobbitt, K. B. Wiberg, J. Org. Chem. 2007, 72, 4504–4509.
- 19The Hammet plot for the oxidation of benzylic primary alcohols (ArCH2OH) did not show linearity.