Distinguishing Alternative Reaction Pathways by Single-Molecule Fluorescence Spectroscopy†
Arina Rybina
Cellnetworks Cluster & Physikalisch-Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 267, 69120 Heidelberg (Germany)
Search for more papers by this authorDr. Carolin Lang
Organisch-Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg (Germany)
Search for more papers by this authorMarcel Wirtz
Biophysikalische Chemie, Universität des Saarlandes, Campus Geb. B2.2, 66123 Saarbrücken (Germany)
Search for more papers by this authorKristin Grußmayer
Cellnetworks Cluster & Physikalisch-Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 267, 69120 Heidelberg (Germany)
Search for more papers by this authorDr. Anton Kurz
Cellnetworks Cluster & Physikalisch-Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 267, 69120 Heidelberg (Germany)
Search for more papers by this authorFrank Maier
Organisch-Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg (Germany)
Search for more papers by this authorDr. Alexander Schmitt
Biophysikalische Chemie, Universität des Saarlandes, Campus Geb. B2.2, 66123 Saarbrücken (Germany)
Search for more papers by this authorProf. Oliver Trapp
Organisch-Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg (Germany)
Search for more papers by this authorProf. Gregor Jung
Biophysikalische Chemie, Universität des Saarlandes, Campus Geb. B2.2, 66123 Saarbrücken (Germany)
Search for more papers by this authorCorresponding Author
Dr. Dirk-Peter Herten
Cellnetworks Cluster & Physikalisch-Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 267, 69120 Heidelberg (Germany)
Cellnetworks Cluster & Physikalisch-Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 267, 69120 Heidelberg (Germany)Search for more papers by this authorArina Rybina
Cellnetworks Cluster & Physikalisch-Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 267, 69120 Heidelberg (Germany)
Search for more papers by this authorDr. Carolin Lang
Organisch-Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg (Germany)
Search for more papers by this authorMarcel Wirtz
Biophysikalische Chemie, Universität des Saarlandes, Campus Geb. B2.2, 66123 Saarbrücken (Germany)
Search for more papers by this authorKristin Grußmayer
Cellnetworks Cluster & Physikalisch-Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 267, 69120 Heidelberg (Germany)
Search for more papers by this authorDr. Anton Kurz
Cellnetworks Cluster & Physikalisch-Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 267, 69120 Heidelberg (Germany)
Search for more papers by this authorFrank Maier
Organisch-Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg (Germany)
Search for more papers by this authorDr. Alexander Schmitt
Biophysikalische Chemie, Universität des Saarlandes, Campus Geb. B2.2, 66123 Saarbrücken (Germany)
Search for more papers by this authorProf. Oliver Trapp
Organisch-Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg (Germany)
Search for more papers by this authorProf. Gregor Jung
Biophysikalische Chemie, Universität des Saarlandes, Campus Geb. B2.2, 66123 Saarbrücken (Germany)
Search for more papers by this authorCorresponding Author
Dr. Dirk-Peter Herten
Cellnetworks Cluster & Physikalisch-Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 267, 69120 Heidelberg (Germany)
Cellnetworks Cluster & Physikalisch-Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 267, 69120 Heidelberg (Germany)Search for more papers by this authorWe thank the Deutsche Forschungsgemeinschaft (DFG) for their financial support (EXC81, SFB623). We also acknowledge Stephen Hashmi (Heidelberg University) for fruitful discussions. Volker Huch is gratefully acknowledged for X-ray crystallography. Michael Schwering and Dominik Brox have continuously supported the project with their expertise in microscopy.
Graphical Abstract
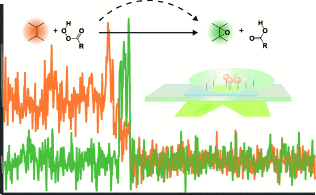
Focus on chemical transitions: Epoxidation of a double bond in conjugation to a fluorescent dye was studied at single-molecule level. Direct observation of oxirane formation, indicated as a spectral shift from substrate to product state, revealed an alternative reaction pathway for the epoxidation reaction.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201300100_sm_miscellaneous_information.pdf1.3 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1S. van de Linde, M. Heilemann, M. Sauer, Ann. Rev. Phys. Chem. 2012, 63, 519–540.
- 2M. A. Thompson, D. L. Matthew, W. E. Moerner, Annu. Rev. Biophys. 2012, 41, 321–342.
- 3D. Wöll, E. Braeken, A. Deres, F. C. De Schryver, H. Uji-i, J. Hofkens, Chem. Soc. Rev. 2009, 38, 313–328.
- 4G. De Cremer, B. F. Sels, D. E. de Vos, J. Hofkens, M. B. J. Roeffaers, Chem. Soc. Rev. 2010, 39, 4703–4717.
- 5F. Kulzer, T. Xia, M. Orrit, Angew. Chem. 2010, 122, 866–879;
10.1002/ange.200904858 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 854–866.
- 6S. Manley, J. M. Gillette, H. Shroff, H. Hess, E. Betzig, J. Lippincott-Schwartz, Nat. Methods 2008, 5, 155–157.
- 7I. L. C. Buurmans, B. M. Weckhuysen, Nat. Chem. 2012, 4, 873–886.
- 8T. Christ, F. Kulzer, P. Bordat, T. Basché, Angew. Chem. 2001, 113, 4323–4326;
10.1002/1521-3757(20011119)113:22<4323::AID-ANGE4323>3.0.CO;2-J Google ScholarAngew. Chem. Int. Ed. 2001, 40, 4192–4195.10.1002/1521-3773(20011119)40:22<4192::AID-ANIE4192>3.0.CO;2-D CAS PubMed Web of Science® Google Scholar
- 9H. P. Lu, L. Xun, X. S. Xie, Science 1998, 282, 1877–1882.
- 10Y. Jiang, N. R. Douglas, N. R. Conley, E. J. Miller, J. Frydman, W. E. Moerner, Proc. Nat. Acad. Sci. USA 2011, 108, 16962–16967.
- 11L. C. Tabares, D. Kostrz, A. Elmalk, A. Andreoni, C. Dennison, T. J. Aartsma, G. W. Canters, Chem. Eur. J. 2011, 17, 12015–12019.
- 12M. B. J. Roeffaers, B. F. Sels, H. Uji-i, F. C. De Schryver, P. A. Jacobs, D. E. De Vos, J. Hofkens, Nature 2006, 439, 572–575.
- 13M. B. J. Roeffaers, G. De Cremer, J. Libeert, R. Ameloot, P. Dedecker, A.-J. Bons, M. Bückins, J. A. Martens, B. F. Sels, D. E. De Vos, J. Hofkens, Angew. Chem. 2009, 121, 9449–9453;
10.1002/ange.200904944 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 9285–9289.
- 14K. Velonia, O. Flomenbom, D. Loos, S. Masuo, M. Cotlet, Y. Engelborghs, J. Hofkens, A. E. Rowan, J. Klafter, R. J. M. Nolte, F. C. de Schryver, Angew. Chem. 2005, 117, 566–570;
10.1002/ange.200460625 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 560–564.
- 15C. Peng, W. Xu, X. Zhou, D. Panda, A. Kalininskiy, Chem. Phys. Lett. 2009, 470, 151–157.
- 16T. Tachikawa, S. Yamashita, T. Majima, Angew. Chem. 2010, 122, 442–445;
10.1002/ange.200904876 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 432–435.
- 17S. M. Canham, J. Y. Bass, O. Navarro, S. Lim, N. Neeladri, S. A. Blum, Organometallics 2008, 27, 2172–2175.
- 18N. M. Esfandiari, S. A. Blum, J. Am. Chem. Soc. 2011, 133, 18145–18147.
- 19A. Kiel, J. Kovacs, A. Mokhir, R. Krämer, D. P. Herten, Angew. Chem. 2007, 119, 3427–3430;
10.1002/ange.200604965 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 3363–3366.
- 20R. Ameloot, M. Roeffaers, M. Baruah, G. De Cremer, B. Sels, D. De Vos, J. Hofkens, Photochem. Photobiol. Sci 2009, 8, 453–456.
- 21G. Jung, A. Schmitt, M. Jacob, B. Hinkeldey, Ann. N. Y. Acad. Sci. 2008, 1130, 131–137.
- 22P. D. Bartlett, Rec. Chem. Prog. 1950, 11, 47–51.
- 23G. De Cremer, M. B. J. Roeffaers, E. Bartholomeeusen, K. Lin, P. Dedecker, P. P. Pescarmona, P. A. Jacobs, D. E. De Vos, J. Hofkens, B. F. Sels, Angew. Chem. 2010, 122, 920–923;
10.1002/ange.200905039 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 908–911.
- 24A. Schmitt, B. Hinkeldey, B. Hötzer, G. Jung, J. Phys. Org. Chem. 2009, 22, 1233–1238.
- 25A. Loudet, K. Burgess, Chem. Rev. 2007, 107, 4891–4932.
- 26G. Ulrich, R. Ziessel, A. Harriman, Angew. Chem. 2008, 120, 1202–1219;
10.1002/ange.200702070 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 1184–1201.
- 27H. Cousin, O. Trapp, V. Peulon-Agasse, X. Pannecoucke, L. Banspach, G. Trapp, Z. Jiang, J. C. Combret, V. Schurig, Eur. J. Org. Chem. 2003, 3273–3287.
- 28C. Lang, U. Gärtner, O. Trapp, Chem. Commun. 2011, 47, 391–393.
- 29M. J. Spallek, G. Storch, O. Trapp, Eur. J. Org. Chem. 2012, 3929–3945.
- 30R. D. Bach, C. Canepa, J. E. Winter, P. E. Blanchette, J. Org. Chem. 1997, 62, 5191–5197.
- 31H. Shi, Z. Zhang, Y. Wang, J. Mol. Catal. A 2005, 238, 13–25.
- 32M. B. J. Roeffaers, G. De Cremer, H. Uji-i, B. Muls, B. F. Sels, P. A. Jacobs, F. C. De Schryver, D. E. De Vos, J. Hofkens, Proc. Nat. Acad. Sci. USA 2007, 104, 12603–12609.