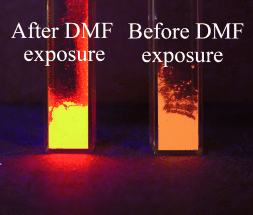
A Luminescent Metal–Organic Framework as a Turn-On Sensor for DMF Vapor†
Yu Li
Department of Chemistry, University of Toronto, 80 St. George Street, ON M5S 3H6 (Canada)
Search for more papers by this authorShanshan Zhang
Department of Chemistry, University of Toronto, 80 St. George Street, ON M5S 3H6 (Canada)
Search for more papers by this authorCorresponding Author
Prof. Datong Song
Department of Chemistry, University of Toronto, 80 St. George Street, ON M5S 3H6 (Canada)
Department of Chemistry, University of Toronto, 80 St. George Street, ON M5S 3H6 (Canada)Search for more papers by this authorYu Li
Department of Chemistry, University of Toronto, 80 St. George Street, ON M5S 3H6 (Canada)
Search for more papers by this authorShanshan Zhang
Department of Chemistry, University of Toronto, 80 St. George Street, ON M5S 3H6 (Canada)
Search for more papers by this authorCorresponding Author
Prof. Datong Song
Department of Chemistry, University of Toronto, 80 St. George Street, ON M5S 3H6 (Canada)
Department of Chemistry, University of Toronto, 80 St. George Street, ON M5S 3H6 (Canada)Search for more papers by this authorThis research is supported by grants to D.S. from the Natural Science and Engineering Research Council (NSERC) of Canada and the Ontario Ministry of Research and Innovation.
Graphical Abstract
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201207610_sm_miscellaneous_information.pdf934.9 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1L. J. Murray, M. Dincă, J. R. Long, Chem. Soc. Rev. 2009, 38, 1294–1314.
- 2J.-R. Li, R. J. Kuppler, H.-C. Zhou, Chem. Soc. Rev. 2009, 38, 1477–1504.
- 3
- 3aJ. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen, J. T. Hupp, Chem. Soc. Rev. 2009, 38, 1450–1459;
- 3bL. Ma, C. Abney, W. Lin, Chem. Soc. Rev. 2009, 38, 1248–1256.
- 4
- 4aB. Chen, Y. Yang, F. Zapata, G. Lin, G. Qian, E. B. Lobkovsky, Adv. Mater. 2007, 19, 1693–1696;
- 4bY. Xiao, Y. Cui, Q. Zheng, S. Xiang, G. Qian, B. Chen, Chem. Commun. 2010, 46, 5503–5505;
- 4cB. Chen, L. Wang, Y. Xiao, F. R. Fronczek, M. Xue, Y. Cui, G. Qian, Angew. Chem. 2009, 121, 508–511; Angew. Chem. Int. Ed. 2009, 48, 500–503;
- 4dB. Chen, L. Wang, F. Zapata, G. Qian, E. B. Lobkovsky, J. Am. Chem. Soc. 2008, 130, 6718–6719;
- 4eB. Zhao, X.-Y. Chen, P. Cheng, D.-Z. Liao, S.-P. Yan, Z.-H. Jiang, J. Am. Chem. Soc. 2004, 126, 15394–15395;
- 4fW.-G. Lu, L. Jiang, X.-L. Feng, T.-B. Lu, Inorg. Chem. 2009, 48, 6997–6999;
- 4gZ.-Z. Lu, R. Zhang, Y.-Z. Li, Z.-J. Guo, H.-G. Zheng, J. Am. Chem. Soc. 2011, 133, 4172–4174;
- 4hA. Lan, K. Li, H. Wu, D. H. Olson, T. J. Emge, W. Ki, M. Hong, J. Li, Angew. Chem. 2009, 121, 2370–2374; Angew. Chem. Int. Ed. 2009, 48, 2334–2338;
- 4iZ. Xie, L. Ma, K. E. deKrafft, A. Jin, W. Lin, J. Am. Chem. Soc. 2010, 132, 922–923;
- 4jJ. An, C. M. Shade, D. A. Chengelis-Czegan, S. Petoud, N. L. Rosi, J. Am. Chem. Soc. 2011, 133, 1220–1223;
- 4kG. Lu, J. T. Hupp, J. Am. Chem. Soc. 2010, 132, 7832–7833;
- 4lY. Takashima, V. M. Martínez, S. Furukawa, M. Kondo, S. Shimomura, H. Uehara, M. Nakahama, K. Sugimoto, S. Kitagawa, Nat. Commun. 2011, 2, 168;
- 4mS. Liu, Z. Xiang, Z. Hu, X. Zheng, D. Cao, J. Mater. Chem. 2011, 21, 6649–6653;
- 4nZ. Guo, H. Xu, S. Su, J. Cai, Dang, S. Xiang, G. Qian, H. Xhang, M. O’Keeffe, B. Chen, Chem. Commun. 2011, 47, 5551–5553.
- 5J. A. Hurd, R. Vaidhyanathan, V. Thangadurai, C. I. Ratcliffe, I. L. Moudrakovski, G. K. H. Shimizu, Nat. Chem. 2009, 1, 705–710.
- 6Y. Cui, H. Xu, Y. Yue, Z. Guo, J. Yu, Z. Chen, J. Gao, Y. Yang, G. Qian, B. Chen, J. Am. Chem. Soc. 2012, 134, 3979–3982.
- 7
- 7aG. Long, M. E. Meek, J. Environ. Sci. Health Part C 2001, 19, 161–187;
- 7bM. Hamada, M. Abe, Y. Tokumoto, T. Miyake, H. Murakami, Y. Hiasa, B. Matsuura, K. Sato, M. Onji, Intern. Med. 2009, 48, 1647–1650.
- 8
- 8aH. Ohbayashi, K. Yamazaki, S. Aiso, K. Nagano, S. Fukushima, H. Ohta, J. Toxicol. Sci. 2008, 33, 327–338;
- 8bR. Ding, D. Chen, Y. Yang, Environ. Toxicol. Pharmacol. 2011, 31, 357–363.
- 9
- 9aA. M. Saillenfait, J. P. Payan, D. Beydon, J. P. Fabry, I. Langonne, J. P. Sabate, F. Gallissot, Fundam. Appl. Toxicol. 1997, 39, 33–43;
- 9bJ. J. Hellwig, J. Merkle, H. J. Klimisch, R. Jäckh, Food Chem. Toxicol. 1991, 29, 193–201.
- 10
- 10aA. M. Ducatman, D. E. Conwill, J. Crawl, J. Urol. 1986, 136, 834–836;
- 10bS. M. Levin, D. B. Baker, P. J. Landrigan, S. V. Monaghan, E. Frumin, M. Braithwaite, W. Towne, Lancet 1987, 8568, 1153–1153;
10.1016/S0140-6736(87)91587-X Google Scholar
- 10cH. Senoh, S. Aiso, H. Arito, T. Nishizawa, K. Nagano, S. Yamamoto, T. Matsushima, J. Occup. Health 2004, 46, 429–439.
- 11
- 11aV. Rimatori, G. Carelli, Scand. J. Work Environ. Health 1982, 8, 20–23;
- 11bE. W. March, L. S. Ettre, Chromatogr. Newsl. 1977, 5, 7–8;
- 11chttp://www.osha.gov/dts/sltc/methods/organic/org066/org066.html.
- 12CCDC 855541 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 13The νCO band of DMF at 1660 cm−1 was not longer evident in the FTIR spectrum of 2 (Figure S7) and nitrogen was no longer detected in elemental analysis. (Anal. calcd for [Eu2L3(H2O)4]⋅13 H2O: C, 47.43; H, 5.20; N, 0. Found: C, 47.26; H, 5.11; N, 0.) Powder X-ray diffraction shows that 2 is crystalline (Figure S8).
- 14All incubated samples except that with formamide reached maximum emission intensity within 24 h; the emission intensity of the formamide-incubated sample continued to increase slowly to 1.3-fold enhancement after 96 h, likely due to its low vapor pressure (11 Pa at 20 °C).
- 15Y. Haas, G. Stein, J. Phys. Chem. 1971, 75, 3677–3681.
- 16The difference between 2 and 3 is that 2 is air dried while 3 is dried under vacuum which is necessary before it can be transfered into the glovebox. Keeping in mind the two water molecules per Eu center in the crystal structure of 2, the 0.5 coordinated water molecules per Eu found in 3-H2O/D2O sample is likely the direct consequence of the drying process.
- 17W. D. Horrocks, Jr., D. R. Sudnick, J. Am. Chem. Soc. 1979, 101, 334–340.
- 18G. Férey, C. Serre, Chem. Soc. Rev. 2009, 38, 1380–1399.
- 19The decease in the intensity in “on” state is not observed for recycled powder samples (Figure S10), suggesting that the slight intensity decrease over many on–off cycles shown in Figure 4 is likely due to the short exposure time or instability of the sensor slides because of engineering problems.
- 20The response rate was found to be sensitive to the experimental setup. The typical ranges are reported here. Refer to the Supporting Information for details regarding the sensing experiments.