Simultaneous Measurement of SUMOylation using SNAP/CLIP-Tag-Mediated Translation at the Single-Molecule Level†
Dr. Yong Yang
Single-molecule Detection and Imaging Laboratory, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Guangdong, 518055 (China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Chun-yang Zhang
Single-molecule Detection and Imaging Laboratory, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Guangdong, 518055 (China)
Single-molecule Detection and Imaging Laboratory, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Guangdong, 518055 (China)Search for more papers by this authorDr. Yong Yang
Single-molecule Detection and Imaging Laboratory, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Guangdong, 518055 (China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Chun-yang Zhang
Single-molecule Detection and Imaging Laboratory, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Guangdong, 518055 (China)
Single-molecule Detection and Imaging Laboratory, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Guangdong, 518055 (China)Search for more papers by this authorThis work was supported by the National Basic Research Program 973 (Grant Nos. 2011CB933600 and 2010CB732600), the Award for the Hundred Talent Program of the Chinese Academy of Science, the Natural Science Foundation of China (Grant Nos. 21075129 and 31000599), the Guangdong Innovation Research Team Fund for Low-cost Healthcare Technologies, the Natural Science Foundation of Shenzhen City (Grant Nos. JC201005270327A and JC201005270355A), and the Fund for Shenzhen Engineering Laboratory of Single-molecule Detection and Instrument Development (Grant No. (2012) 433). SUMO=small ubiquitin-like modifier.
Graphical Abstract
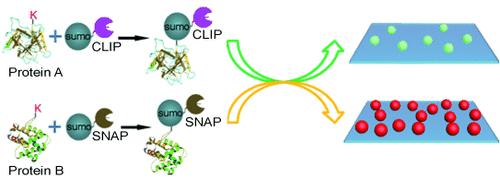
Two opposing SUMO wrestlers: Simultaneous measurement of multiple SUMOylations was achieved at the single-molecule level by integrating SNAP/CLIP-tag-mediated translation with single-molecule detection (see scheme; SUMO=small ubiquitin-like modifier). This method gives exceptional sensitivity, and is capable of measuring the SUMOylation of different proteins under various physiological conditions.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201206695_sm_miscellaneous_information.pdf394.1 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1E. S. Johnson, Annu. Rev. Biochem. 2004, 73, 355–382.
- 2S. Creton, S. Jentsch, Cell 2010, 143, 848–848.e1.
- 3S. Muller, C. Hoege, G. Pyrowolakis, S. Jentsch, Nat. Rev. Mol. Cell Biol. 2001, 2, 202–210.
- 4F. S. Alkuraya, I. Saadi, J. J. Lund, A. Turbe-Doan, C. C. Morton, R. L. Maas, Science 2006, 313, 1751–1751.
- 5K. I. Kim, S. H. Baek, Mol. Cells 2006, 22, 247–253.
- 6K. D. Sarge, O. K. Park-Sarge, Trends Biochem. Sci. 2009, 34, 200–205.
- 7J. S. Steffan, N. Agrawal, J. Pallos, E. Rockabrand, L. C. Trotman, N. Slepko, K. Illes, T. Lukacsovich, Y. Z. Zhu, E. Cattaneo, P. P. Pandolfi, L. M. Thompson, J. L. Marsh, Science 2004, 304, 100–104.
- 8M. Li, D. Guo, C. M. Isales, D. L. Eizirik, M. Atkinson, J. X. She, C. Y. Wang, J. Mol. Med. 2005, 83, 504–513.
- 9J. Wang, R. J. Schwartz, Circ. Res. 2010, 107, 19–29.
- 10R. Geiss-Friedlander, F. Melchior, Nat. Rev. Mol. Cell Biol. 2007, 8, 947–956.
- 11S. Sommer, N. D. Weikart, A. Brockmeyer, P. Janning, H. D. Mootz, Angew. Chem. 2011, 123, 10062–10066;
10.1002/ange.201102531 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 9888–9892.
- 12F. Golebiowski, I. Matic, M. H. Tatham, C. Cole, Y. Yin, A. Nakamura, J. Cox, G. J. Barton, M. Mann, R. T. Hay, Sci. Signaling 2009, 2, ra 24.
- 13A. Jakobs, J. Koehnke, F. Himstedt, M. Funk, B. Korn, M. Gaestel, R. Niedenthal, Nat. Methods 2007, 4, 245–250.
- 14A. Verger, J. Perdomo, M. Crossley, EMBO Rep. 2003, 4, 137–142.
- 15G. Bossis, C. E. Malnou, R. Farras, E. Andermarcher, R. Hipskind, M. Rodriguez, D. Schmidt, S. Muller, I. Jariel-Encontre, M. Piechaczyk, Mol. Cell. Biol. 2005, 25, 6964–6979.
- 16S. Bergink, S. Jentsch, Nature 2009, 458, 461–467.
- 17J. Joseph, S. H. Tan, T. S. Karpova, J. G. McNally, M. Dasso, J. Cell Biol. 2002, 156, 595–602.
- 18P. R. Potts, H. Yu, Nat. Struct. Mol. Biol. 2007, 14, 581–590.
- 19L. E. Hang, X. Liu, I. Cheung, Y. Yang, X. Zhao, Nat. Struct. Mol. Biol. 2011, 18, 920–926.
- 20X. D. Zhang, J. Goeres, H. Zhang, T. J. Yen, A. C. Porter, M. J. Matunis, Mol. Cell 2008, 29, 729–741.
- 21J. S. Seeler, A. Dejean, Nat. Rev. Mol. Cell Biol. 2003, 4, 690–699.
- 22Y. Xue, F. Zhou, C. Fu, Y. Xu, X. Yao, Nucleic Acids Res. 2006, 34, W 254–W257.
- 23Q. Dumont, D. L. Donaldson, W. P. Griffith, Anal. Chem. 2011, 83, 9638–9642.
- 24P. G. Pedrioli, B. Raught, X. D. Zhang, R. Rogers, J. Aitchison, M. Matunis, R. Aebersold, Nat. Methods 2006, 3, 533–539.
- 25F. Galisson, L. Mahrouche, M. Courcelles, E. Bonneil, S. Meloche, M. K. Chelbi-Alix, P. Thibault, Mol. Cell. Proteomics 2010, 10, M 110 004796.
- 26A. Jakobs, F. Himstedt, M. Funk, B. Korn, M. Gaestel, R. Niedenthal, Nucleic Acids Res. 2007, 35, e 109.
- 27R. Niedenthal, Methods Mol. Biol. 2009, 497, 63–79.
- 28C. Jing, V. W. Cornish, Acc. Chem. Res. 2011, 44, 784–792.
- 29M. J. Hinner, K. Johnsson, Curr. Opin. Biotechnol. 2010, 21, 766–776.
- 30A. Keppler, S. Gendreizig, T. Gronemeyer, H. Pick, H. Vogel, K. Johnsson, Nat. Biotechnol. 2003, 21, 86–89.
- 31A. Juillerat, T. Gronemeyer, A. Keppler, S. Gendreizig, H. Pick, H. Vogel, K. Johnsson, Chem. Biol. 2003, 10, 313–317.
- 32A. Gautier, A. Juillerat, C. Heinis, I. R. Correa, Jr., M. Kindermann, F. Beaufils, K. Johnsson, Chem. Biol. 2008, 15, 128–136.
- 33B. Mollwitz, E. Brunk, S. Schmitt, F. Pojer, M. Bannwarth, M. Schiltz, U. Rothlisberger, K. Johnsson, Biochemistry 2012, 51, 986–994.
- 34K. Bojkowska, F. Santoni de Sio, I. Barde, S. Offner, S. Verp, C. Heinis, K. Johnsson, D. Trono, Chem. Biol. 2011, 18, 805–815.
- 35D. Toomre, Cold Spring Harb. Protoc. 2012, 414–424.
- 36Y. Yang, C. Y. Zhang, Anal. Chem. 2012, 84, 1229–1234.
- 37D. J. Crawford, A. A. Hoskins, L. J. Friedman, J. Gelles, M. J. Moore, RNA 2008, 14, 170–179.
- 38S. Muller, M. Berger, F. Lehembre, J. S. Seeler, Y. Haupt, A. Dejean, J. Biol. Chem. 2000, 275, 13321–13329.
- 39T. Li, R. Santockyte, R. F. Shen, E. Tekle, G. Wang, D. C. Yang, P. B. Chock, J. Biol. Chem. 2006, 281, 36221–36227.
- 40J. Herrmann, L. O. Lerman, A. Lerman, Circ. Res. 2007, 100, 1276–1291.
- 41M. Hochstrasser, Nature 2009, 458, 422–429.
- 42C. Fu, K. Ahmed, H. Ding, X. Ding, J. Lan, Z. Yang, Y. Miao, Y. Zhu, Y. Shi, J. Zhu, H. Huang, X. Yao, Oncogene 2005, 24, 5401–5413.
- 43H. M. Chan, L. S. Chan, R. N. Wong, H. W. Li, Anal. Chem. 2010, 82, 6911–6918.