Enantioselective Hydrogenation of α-Substituted Acrylic Acids Catalyzed by Iridium Complexes with Chiral Spiro Aminophosphine Ligands†
Prof. Shou-Fei Zhu
State Key Laboratory and Institute of Elemento-organic Chemistry, Nankai University, Tianjin 300071 (China) http://zhou.nankai.edu.cn/
Search for more papers by this authorYan-Bo Yu
State Key Laboratory and Institute of Elemento-organic Chemistry, Nankai University, Tianjin 300071 (China) http://zhou.nankai.edu.cn/
Search for more papers by this authorShen Li
State Key Laboratory and Institute of Elemento-organic Chemistry, Nankai University, Tianjin 300071 (China) http://zhou.nankai.edu.cn/
Search for more papers by this authorLi-Xin Wang
State Key Laboratory and Institute of Elemento-organic Chemistry, Nankai University, Tianjin 300071 (China) http://zhou.nankai.edu.cn/
Search for more papers by this authorCorresponding Author
Prof. Qi-Lin Zhou
State Key Laboratory and Institute of Elemento-organic Chemistry, Nankai University, Tianjin 300071 (China) http://zhou.nankai.edu.cn/
State Key Laboratory and Institute of Elemento-organic Chemistry, Nankai University, Tianjin 300071 (China) http://zhou.nankai.edu.cn/Search for more papers by this authorProf. Shou-Fei Zhu
State Key Laboratory and Institute of Elemento-organic Chemistry, Nankai University, Tianjin 300071 (China) http://zhou.nankai.edu.cn/
Search for more papers by this authorYan-Bo Yu
State Key Laboratory and Institute of Elemento-organic Chemistry, Nankai University, Tianjin 300071 (China) http://zhou.nankai.edu.cn/
Search for more papers by this authorShen Li
State Key Laboratory and Institute of Elemento-organic Chemistry, Nankai University, Tianjin 300071 (China) http://zhou.nankai.edu.cn/
Search for more papers by this authorLi-Xin Wang
State Key Laboratory and Institute of Elemento-organic Chemistry, Nankai University, Tianjin 300071 (China) http://zhou.nankai.edu.cn/
Search for more papers by this authorCorresponding Author
Prof. Qi-Lin Zhou
State Key Laboratory and Institute of Elemento-organic Chemistry, Nankai University, Tianjin 300071 (China) http://zhou.nankai.edu.cn/
State Key Laboratory and Institute of Elemento-organic Chemistry, Nankai University, Tianjin 300071 (China) http://zhou.nankai.edu.cn/Search for more papers by this authorWe thank the National Natural Science Foundation of China and the National Basic Research Program of China (2011CB808600, 2012CB821600), and the “111” project (B06005) of the Ministry of Education of China for financial support.
Graphical Abstract
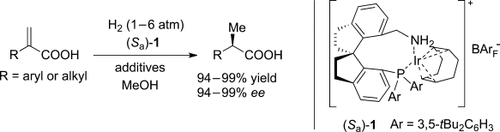
Highly active: Iridium complexes with chiral spiro aminophosphine ligands were synthesized and applied as catalysts for the asymmetric hydrogenation of α-substituted acrylic acids (see scheme). The complexes were highly active catalysts, showing turnover frequencies of up to 6000 h−1, and catalyst loadings could be reduced to 0.01 mol %.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201204363_sm_miscellaneous_information.pdf2.9 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews, see:
- 1aW. S. Knowles, R. Noyori, Acc. Chem. Res. 2007, 40, 1238–1239;
- 1b Handbook of Homogeneous Hydrogenation (Eds.: ), Wiley-VCH, Weinheim, 2007;
- 1c Asymmetric Catalysis on Industrial Scale: Challenges, Approaches and Solutions (Eds.: ), Wiley-VCH, Weinheim, 2004;
- 1dW. Tang, X. Zhang, Chem. Rev. 2003, 103, 3029–3069.
- 2
- 2aC. C. Bausch, A. Pfaltz, Privileged Chiral Ligands and Catalysts (Ed.: ), Wiley-VCH, Weinheim, 2011, Chap. 6, pp. 221–256;
10.1002/9783527635207.ch6 Google Scholar
- 2bS. J. Roseblade, A. Pfaltz, Acc. Chem. Res. 2007, 40, 1402–1411;
- 2cP. J. Guiry, C. P. Saunder, Adv. Synth. Catal. 2004, 346, 497–537.
- 3
- 3aT. Ikariya, K. Murata, R. Noyori, Org. Biomol. Chem. 2006, 4, 393–406;
- 3bJ.-B. Xie, J.-H. Xie, X.-Y. Liu, W.-L. Kong, S. Li, Q.-L. Zhou, J. Am. Chem. Soc. 2010, 132, 4538–4539.
- 4For the preparation of intermediate 4, see: J.-H. Xie, L.-X. Wang, Y. Fu, S.-F. Zhu, B.-M. Fan, H.-F. Duan, Q.-L. Zhou, J. Am. Chem. Soc. 2003, 125, 4404–4405.
- 5CCDC 888308 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 6
- 6aD. Lednicer, L. A. Mitscher, The Organic Chemistry of Drug Synthesis, Vol. 1, Wiley, New York, 1977; D. Lednicer, L. A. Mitscher, The Organic Chemistry of Drug Synthesis, Vol. 2, Wiley, New York, 1980;
- 6bT. Y. Shen, Angew. Chem. 1972, 84, 512–526; Angew. Chem. Int. Ed. Engl. 1972, 11, 460–472;
- 6cP.-J. Harrington, E. Lodewijk, Org. Process Res. Dev. 1997, 1, 72–76.
- 7For representative examples, see:
- 7aT. Ohta, H. Takaya, M. Kitamura, K. Nagai, R. Noyori, J. Org. Chem. 1987, 52, 3174–3176;
- 7bX. Zhang, T. Uemura, K. Matsumura, N. Sayo, H. Kumobayashi, H. Takaya, Synlett 1994, 501–503;
- 7cT. Uemura, X. Zhang, K. Matsumura, N. Sayo, H. Kumobayashi, T. Ohta, K. Nozaki, H. Takaya, J. Org. Chem. 1996, 61, 5510–5516;
- 7dT. Benincori, E. Brenna, F. Sannicolò, L. Trimarco, P. Antognazza, E. Cesarotti, F. Demartin, T. Pilati, J. Org. Chem. 1996, 61, 6244–6251;
- 7eQ.-H. Fan, C.-Y. Ren, C.-H. Yeung, W.-H. Hu, A. S. C. Chan, J. Am. Chem. Soc. 1999, 121, 7407–7408;
- 7fC.-C. Pai, C.-W. Lin, C.-C. Lin, C.-C. Chen, A. S. C. Chan, J. Am. Chem. Soc. 2000, 122, 11513–11514;
- 7gQ.-H. Fan, Y.-M. Chen, X.-M. Chen, D.-Z. Jiang, F. Xi, A. S. C. Chan, Chem. Commun. 2000, 789–790;
- 7hR. A. Brown, P. Pollet, E. McKoon, C. A. Eckert, C. L. Liotta, P. G. Jessop, J. Am. Chem. Soc. 2001, 123, 1254–1255;
- 7iL. Qiu, J. Qi, C.-C. Pai, S. Chan, Z. Zhou, M. C. K. Choi, A. S. C. Chan, Org. Lett. 2002, 4, 4599–4602;
- 7jL. Qiu, J. Wu, S. Chan, T. T.-L. Au-Yeung, J.-X. Ji, R. Guo, C.-C. Pai, Z. Zhou, X. Li, Q.-H. Fan, A. S. C. Chan, Proc. Natl. Acad. Sci. USA 2004, 101, 5815–5820;
- 7kG.-J. Deng, B. Yi, Y.-Y. Huang, W.-J. Tang, Y.-M. He, Q.-H. Fan, Adv. Synth. Catal. 2004, 346, 1440–1444;
- 7lL. Qiu, F. Y. Kwong, J. Wu, W. H. Lam, S. Chan, W.-Y. Yu, Y.-M. Li, R. Guo, Z. Zhou, A. S. C. Chan, J. Am. Chem. Soc. 2006, 128, 5955–5965;
- 7mL. Qiu, Y.-M. Li, F. Y. Kwong, W.-Y. Yu, Q.-H. Fan, A. S. C. Chan, Adv. Synth. Catal. 2007, 349, 517–520.
- 8
- 8aF. Robin, F. Mercier, L. Ricard, F. Mathey, M. Spagnol, Chem. Eur. J. 1997, 3, 1365–1369;
- 8bW.-H. Hu, C. C. Pai, C. C. Chen, G.-P. Xue, A. S. C. Chan, Tetrahedron: Asymmetry 1998, 9, 3241–3246;
- 8cB. Zupančič, B. Mohar, M. Stephan, Adv. Synth. Catal. 2008, 350, 2024–2032;
- 8dM. Stephan, D. Šterk, B. Mohar, Adv. Synth. Catal. 2009, 351, 2779–2786;
- 8eB. Zupančič, B. Mohar, M. Stephan, Org. Lett. 2010, 12, 1296–1299;
- 8fB. Zupančič, B. Mohar, M. Stephan, Org. Lett. 2010, 12, 3022–3025;
- 8gM. Stephan, D. Šterk, B. Zupančič, B. Mohar, Org. Biomol. Chem. 2011, 9, 5266–5271.
- 9Y. Zhang, Z.-B. Han, F.-Y. Li, K.-L. Ding, A. Zhang, Chem. Commun. 2010, 46, 156–158.
- 10A. Scrivanti, S. Bovo, A. Ciappa, U. Matteoli, Tetrahedron Lett. 2006, 47, 9261–9265 and references therein.
- 11For ruthenium/diphosphine-catalyzed asymmetric hydrogenation of trisubstituted α,β-unsaturated carboxylic acids, see:
- 11aX. Cheng, Q. Zhang, J.-H. Xie, L.-X. Wang, Q.-L. Zhou, Angew. Chem. 2005, 117, 1142–1145; Angew. Chem. Int. Ed. 2005, 44, 1118–1121;
- 11bX. Cheng, J.-H. Xie, S. Li, Q.-L. Zhou, Adv. Synth. Catal. 2006, 348, 1271–1276. For iridium/phosphine oxazoline catalyzed asymmetric hydrogenation of trisubstituted α,β- and β,γ-unsaturated carboxylic acids, see:
- 11cS. Li, S.-F. Zhu, C.-M. Zhang, S. Song, Q.-L. Zhou, J. Am. Chem. Soc. 2008, 130, 8584–8585;
- 11dS. Li, S.-F. Zhu, J.-H. Xie, S. Song, C.-M. Zhang, Q.-L. Zhou, J. Am. Chem. Soc. 2010, 132, 1172–1179;
- 11eS. Song, S.-F. Zhu, S. Yang, S. Li, Q.-L. Zhou, Angew. Chem. 2012, 124, 2762–2765; Angew. Chem. Int. Ed. 2012, 51, 2708–2711.
- 12S.-F. Zhu, J.-B. Xie, Y.-Z. Zhang, S. Li, Q.-L. Zhou, J. Am. Chem. Soc. 2006, 128, 12886–12891.
- 13
- 13aM. C. Perry, X. Cui, M. T. Powell, D.-R. Hou, J. H. Reibenspies, K. Burgess, J. Am. Chem. Soc. 2003, 125, 113–123;
- 13bJ. Mazuela, J. J. Verendel, M. Coll, B. Schäffner, A. Börner, P. G. Andersson, O. Pàmies, M. Diéguez, J. Am. Chem. Soc. 2009, 131, 12344–12353.