Organocatalytic Asymmetric Direct C H Functionalization of Ethers: A Highly Efficient Approach to Chiral Spiroethers†
H Functionalization of Ethers: A Highly Efficient Approach to Chiral Spiroethers†
Zhi-Wei Jiao
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou 730000 (P.R. China)
Search for more papers by this authorDr. Shu-Yu Zhang
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou 730000 (P.R. China)
Search for more papers by this authorProf. Chuan He
Department of Chemistry, University of Chicago, 929 East 57th Street, Chicago, IL 60637 (USA)
Search for more papers by this authorCorresponding Author
Prof. Yong-Qiang Tu
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou 730000 (P.R. China)
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou 730000 (P.R. China)Search for more papers by this authorProf. Shao-Hua Wang
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou 730000 (P.R. China)
Search for more papers by this authorProf. Fu-Min Zhang
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou 730000 (P.R. China)
Search for more papers by this authorDr. Yong-Qiang Zhang
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou 730000 (P.R. China)
Search for more papers by this authorHui Li
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou 730000 (P.R. China)
Search for more papers by this authorZhi-Wei Jiao
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou 730000 (P.R. China)
Search for more papers by this authorDr. Shu-Yu Zhang
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou 730000 (P.R. China)
Search for more papers by this authorProf. Chuan He
Department of Chemistry, University of Chicago, 929 East 57th Street, Chicago, IL 60637 (USA)
Search for more papers by this authorCorresponding Author
Prof. Yong-Qiang Tu
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou 730000 (P.R. China)
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou 730000 (P.R. China)Search for more papers by this authorProf. Shao-Hua Wang
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou 730000 (P.R. China)
Search for more papers by this authorProf. Fu-Min Zhang
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou 730000 (P.R. China)
Search for more papers by this authorDr. Yong-Qiang Zhang
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou 730000 (P.R. China)
Search for more papers by this authorHui Li
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou 730000 (P.R. China)
Search for more papers by this authorThis work was supported by the NSFC (Nos. 21072085, 20921120404, 21102061, and 20972059), MOST (“973” Program, No. 2010CB833203), Key National S&T Program “Major New Drug Development” of the Ministry of Health of China (2012ZX09201101-003), and MOE (“111” Program and scholarship award for excellent doctoral student, No. 86007).
Graphical Abstract
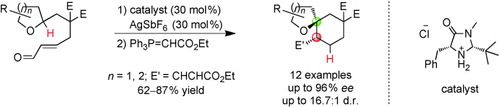
Spiro compounds: An organocatalytic asymmetric method for the C H functionalization of the α position of racemic cyclic ethers has been developed. The transformation, mediated by catalytic amounts of an imidazolidinone and strong acid, involves a tandem 1,5-hydride transfer/cyclization and provides access to a structurally diverse series of chiral spiroethers with high levels of enantioselectivity (see scheme).
H functionalization of the α position of racemic cyclic ethers has been developed. The transformation, mediated by catalytic amounts of an imidazolidinone and strong acid, involves a tandem 1,5-hydride transfer/cyclization and provides access to a structurally diverse series of chiral spiroethers with high levels of enantioselectivity (see scheme).
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201204274_sm_miscellaneous_information.pdf5.8 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aS. Nozoe, M. Morisaki, K. Tsuda, Y. Iitaka, N. Takahashi, S. Tamura, K. Ishibashi, M. Shirasaka, J. Am. Chem. Soc. 1965, 87, 4968–4970;
- 1bM. Entzeroth, A. J. Blackman, J. S. Mynderse, R. E. Moore, J. Org. Chem. 1985, 50, 1255–1259;
- 1cY. Hirasawa, H. Morita, M. Shiro, J. i. Kobayashi, Org. Lett. 2003, 5, 3991–3993;
- 1dS. Aoki, Y. Watanabe, M. Sanagawa, A. Setiawan, N. Kotoku, M. Kobayashi, J. Am. Chem. Soc. 2006, 128, 3148–3149;
- 1eF. A. Macías, J. L. G. Galindo, R. M. Varela, A. Torres, J. M. G. Molinillo, F. R. Fronczek, Org. Lett. 2006, 8, 4513–4516;
- 1fI. A. Katsoulis, G. Kythreoti, A. Papakyriakou, K. Koltsida, P. Anastasopoulou, C. I. Stathakis, I. Mavridis, T. Cottin, E. Saridakis, D. Vourloumis, ChemBioChem 2011, 12, 1188–1192.
- 2
- 2aR. E. Ireland, P. Maienfisch, J. Org. Chem. 1988, 53, 640–651;
- 2bJ. T. Negri, L. A. Paquette, J. Am. Chem. Soc. 1992, 114, 8835–8841;
- 2cM. D. Lord, J. T. Negri, L. A. Paquette, J. Org. Chem. 1995, 60, 191–195;
- 2dN. Haddad, I. Rukhman, Z. Abramovich, J. Org. Chem. 1997, 62, 7629–7636;
- 2eL. A. Paquette, D. R. Owen, R. T. Bibart, C. K. Seekamp, A. L. Kahane, J. C. Lanter, M. A. Corral, J. Org. Chem. 2001, 66, 2828–2834;
- 2fX. Teng, D. R. Cefalo, R. R. Schrock, A. H. Hoveyda, J. Am. Chem. Soc. 2002, 124, 10779–10784;
- 2gN. Noguchi, M. Nakada, Org. Lett. 2006, 8, 2039–2042;
- 2hM. Lejkowski, P. Banerjee, J. Runsink, H.-J. Gais, Org. Lett. 2008, 10, 2713–2716;
- 2iQ.-W. Zhang, C.-A. Fan, H.-J. Zhang, Y.-Q. Tu, Y.-M. Zhao, P. Gu, Z.-M. Chen, Angew. Chem. 2009, 121, 8724–8726; Angew. Chem. Int. Ed. 2009, 48, 8572–8574.
- 3Selected reviews on C
 H functionalizations:
H functionalizations:
- 3aH. M. L. Davies, Angew. Chem. 2006, 118, 6574–6577;
10.1002/ange.200601814 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 6422–6425;
- 3bR. G. Bergman, Nature 2007, 446, 391–393;
- 3cH. M. L. Davies, J. R. Manning, Nature 2008, 451, 417–424;
- 3dX. Chen, K. M. Engle, D.-H. Wang, J.-Q. Yu, Angew. Chem. 2009, 121, 5196–5217; Angew. Chem. Int. Ed. 2009, 48, 5094–5115;
- 3eR. Jazzar, J. Hitce, A. Renaudat, J. Sofack-Kreutzer, O. Baudoin, Chem. Eur. J. 2010, 16, 2654–2672;
- 3fC. S. Yeung, V. M. Dong, Chem. Rev. 2011, 111, 1215–1292;
- 3gC.-L. Sun, B.-J. Li, Z.-J. Shi, Chem. Rev. 2010, 111, 1293–1314;
- 3hC. Liu, H. Zhang, W. Shi, A. Lei, Chem. Rev. 2011, 111, 1780–1824;
- 3iS.-Y. Zhang, F.-M. Zhang, Y.-Q. Tu, Chem. Soc. Rev. 2011, 40, 1937–1949;
- 3jL. McMurray, F. O’Hara, M. J. Gaunt, Chem. Soc. Rev. 2011, 40, 1885–1898;
- 3kC.-M. Che, V. K.-Y. Lo, C.-Y. Zhou, J.-S. Huang, Chem. Soc. Rev. 2011, 40, 1950–1975;
- 3lW. R. Gutekunst, P. S. Baran, Chem. Soc. Rev. 2011, 40, 1976–1991;
- 3mJ. F. Hartwig, Chem. Soc. Rev. 2011, 40, 1992;
- 3nP. Herrmann, T. Bach, Chem. Soc. Rev. 2011, 40, 2022–2038.
- 4
- 4aB. M. Trost, Science 1991, 254, 1471–1477;
- 4bP. A. Wender, F. C. Bi, G. G. Gamber, F. Gosselin, R. D. Hubbard, M. J. C. Scanio, R. Sun, T. J. Williams, L. Zhang, Pure Appl. Chem. 2002, 74, 25–31.
- 5For selected recent reports of reactions involving a 1,5-hydride shift, see:
- 5aW. H. N. Nijhuis, W. Verboom, D. N. Reinhoudt, S. Harkema, J. Am. Chem. Soc. 1987, 109, 3136–3138;
- 5bS. J. Pastine, K. M. McQuaid, D. Sames, J. Am. Chem. Soc. 2005, 127, 12180–12181;
- 5cS. J. Pastine, D. Sames, Org. Lett. 2005, 7, 5429–5431;
- 5dM. Tobisu, N. Chatani, Angew. Chem. 2006, 118, 1713–1715;
10.1002/ange.200503866 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 1683–1684;
- 5eG. B. Bajracharya, N. K. Pahadi, I. D. Gridnev, Y. Yamamoto, J. Org. Chem. 2006, 71, 6204–6210;
- 5fJ. Barluenga, M. Fañanás-Mastral, F. Aznar, C. Valdés, Angew. Chem. 2008, 120, 6696–6699;
10.1002/ange.200802268 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 6594–6597;
- 5gK. M. McQuaid, D. Sames, J. Am. Chem. Soc. 2009, 131, 402–403;
- 5hS. Murarka, C. Zhang, M. D. Konieczynska, D. Seidel, Org. Lett. 2009, 11, 129–132;
- 5iD. Shikanai, H. Murase, T. Hata, H. Urabe, J. Am. Chem. Soc. 2009, 131, 3166–3167;
- 5jJ. C. Ruble, A. R. Hurd, T. A. Johnson, D. A. Sherry, M. R. Barbachyn, P. L. Toogood, G. L. Bundy, D. R. Graber, G. M. Kamilar, J. Am. Chem. Soc. 2009, 131, 3991–3997;
- 5kM. Tobisu, H. Nakai, N. Chatani, J. Org. Chem. 2009, 74, 5471–5475;
- 5lS. Yang, Z. Li, X. Jian, C. He, Angew. Chem. 2009, 121, 4059–4061; Angew. Chem. Int. Ed. 2009, 48, 3999–4001;
- 5mS. J. Mahoney, D. T. Moon, J. Hollinger, E. Fillion, Tetrahedron Lett. 2009, 50, 4706–4709;
- 5nP. A. Vadola, D. Sames, J. Am. Chem. Soc. 2009, 131, 16525–16528;
- 5oK. Mori, T. Kawasaki, S. Sueoka, T. Akiyama, Org. Lett. 2010, 12, 1732–1735;
- 5pI. D. Jurberg, Y. Odabachian, F. Gagosz, J. Am. Chem. Soc. 2010, 132, 3543–3552;
- 5qB. Bolte, Y. Odabachian, F. Gagosz, J. Am. Chem. Soc. 2010, 132, 7294–7296;
- 5rG. Zhou, J. Zhang, Chem. Commun. 2010, 46, 6593–6595;
- 5sM. Alajarin, B. Bonillo, M.-M. Ortin, P. Sanchez-Andrada, A. Vidal, R.-A. Orenes, Org. Biomol. Chem. 2010, 8, 4690–4700;
- 5tM. Alajarin, B. Bonillo, M.-M. Ortin, P. Sanchez-Andrada, A. Vidal, Eur. J. Org. Chem. 2011, 1896–1913;
- 5uK. Mori, S. Sueoka, T. Akiyama, J. Am. Chem. Soc. 2011, 133, 2424–2426;
- 5vB. Bolte, F. Gagosz, J. Am. Chem. Soc. 2011, 133, 7696–7699;
- 5wF. Cambeiro, S. López, J. A. Varela, C. Saá, Angew. Chem. 2012, 124, 747–751;
10.1002/ange.201107344 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 723–727;
- 5xI. D. Jurberg, B. Peng, E. Wöstefeld, M. Wasserloos, N. Maulide, Angew. Chem. 2012, 124, 1986–1989;
10.1002/ange.201108639 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 1950–1953.
- 6Y. K. Kang, S. M. Kim, D. Y. Kim, J. Am. Chem. Soc. 2010, 132, 11847–11849.
- 7For reports of asymmetric reactions that have been classified under the term “tert-amino effect”, see:
- 7aS. Murarka, I. Deb, C. Zhang, D. Seidel, J. Am. Chem. Soc. 2009, 131, 13226–13227;
- 7bW. Cao, X. Liu, W. Wang, L. Lin, X. Feng, Org. Lett. 2011, 13, 600–603;
- 7cG. Zhou, F. Liu, J. Zhang, Chem. Eur. J. 2011, 17, 3101–3104;
- 7dK. Mori, K. Ehara, K. Kurihara, T. Akiyama, J. Am. Chem. Soc. 2011, 133, 6166–6169;
- 7eY. P. He, Y. L. Du, S. W. Luo, L. Z. Gong, Tetrahedron Lett. 2011, 52, 7064–7066;
- 7fL. J. Chen, L. Zhang, J. Lv, J. P. Cheng, S. Z. Luo, Chem. Eur. J. 2012, 18, 8891-8895.
- 8For selected reviews on organocatalysis, see:
- 8aD. Enders, C. Grondal, M. R. M. Hüttl, Angew. Chem. 2007, 119, 1590–1601;
10.1002/ange.200603129 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 1570–1581;
- 8bD. W. C. MacMillan, Nature 2008, 455, 304–308;
- 8cS. Bertelsen, K. A. Jørgensen, Chem. Soc. Rev. 2009, 38, 2178–2189;
- 8dC. Grondal, M. Jeanty, D. Enders, Nat. Chem. 2010, 2, 167–178.
- 9
- 9aK. A. Ahrendt, C. J. Borths, D. W. C. MacMillan, J. Am. Chem. Soc. 2000, 122, 4243–4244;
- 9bS. G. Ouellet, J. B. Tuttle, D. W. C. MacMillan, J. Am. Chem. Soc. 2004, 126, 32–33;
- 9cY. Huang, A. M. Walji, C. H. Larsen, D. W. C. MacMillan, J. Am. Chem. Soc. 2005, 127, 15051–15053;
- 9dJ. W. Yang, M. T. Hechavarria Fonseca, B. List, J. Am. Chem. Soc. 2005, 127, 15036–15037.
- 10The obtained aldehydes 2′′ were not sufficiently stable for HPLC analysis.
- 11
- 11aD. M. Van Seggen, P. K. Hurlburt, M. D. Noirot, O. P. Anderson, S. H. Strauss, Inorg. Chem. 1992, 31, 1423–1430;
- 11bM. Brookhart, B. Grant, A. F. Volpe, Organometallics 1992, 11, 3920–3922;
- 11cY. Hayashi, J. J. Rohde, E. J. Corey, J. Am. Chem. Soc. 1996, 118, 5502–5503;
- 11dL. L. Anderson, J. Arnold, R. G. Bergman, J. Am. Chem. Soc. 2005, 127, 14542–14543;
- 11eC. Böing, G. Franciò, W. Leitner, Adv. Synth. Catal. 2005, 347, 1537–1541;
- 11fS. L. Dabb, J. H. H. Ho, R. Hodgson, B. A. Messerle, J. Wagler, Dalton Trans. 2009, 634–642;
- 11gS. F. Rach, F. E. Kühn, Chem. Rev. 2009, 109, 2061–2080.
- 12Under the standard reaction conditions, a substrate containing benzofuran ring did not give the product:
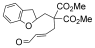
- 13CCDC 883142 (5 a) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 14I. K. Mangion, A. B. Northrup, D. W. C. MacMillan, Angew. Chem. 2004, 116, 6890–6892;
10.1002/ange.200461851 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 6722–6724.
- 15To ensure there was no excess AgSbF6, which could lead to the decomposition of substrate and erode product d.r. and ee value, an additional 1 mg of catalyst 7 was added to the mixture.