Urea/Transition-Metal Cooperative Catalyst for anti-Selective Asymmetric Nitroaldol Reactions†
Kai Lang
Department of Chemistry, University of Florida, Gainesville, FL 32611-7200 (USA)
Search for more papers by this authorJongwoo Park
Department of Chemistry, University of Florida, Gainesville, FL 32611-7200 (USA)
Search for more papers by this authorCorresponding Author
Prof. Sukwon Hong
Department of Chemistry, University of Florida, Gainesville, FL 32611-7200 (USA)
Department of Chemistry, University of Florida, Gainesville, FL 32611-7200 (USA)Search for more papers by this authorKai Lang
Department of Chemistry, University of Florida, Gainesville, FL 32611-7200 (USA)
Search for more papers by this authorJongwoo Park
Department of Chemistry, University of Florida, Gainesville, FL 32611-7200 (USA)
Search for more papers by this authorCorresponding Author
Prof. Sukwon Hong
Department of Chemistry, University of Florida, Gainesville, FL 32611-7200 (USA)
Department of Chemistry, University of Florida, Gainesville, FL 32611-7200 (USA)Search for more papers by this authorThis work was supported by the U.S. National Science Foundation (Grant CHE-0957643).
Graphical Abstract
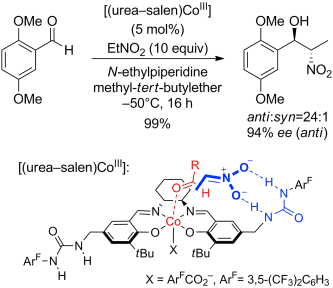
A cooperative catalyst that features urea H-bonding and a cobalt center was developed for anti-selective asymmetric Henry reactions (see scheme). The H-bonds of urea play a crucial role in the improvement in yield (from 30 % to 84 %), enantioselectivity (from 78 % to 96 %), and anti diastereoselectivity (from 3:1 to 48:1). A short synthesis of (1R,2S)-methoxamine hydrochloride was also accomplished with this catalyst.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201107785_sm_miscellaneous_information.pdf3.4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Reviews:
- 1aJ.-A. Ma, D. Cahard, Angew. Chem. 2004, 116, 4666; Angew. Chem. Int. Ed. 2004, 43, 4566;
- 1bD. H. Paull, C. J. Abraham, M. T. Scerba, E. Alden-Danforth, T. Lectka, Acc. Chem. Res. 2008, 41, 655;
- 1cM. Shibasaki, M. Kanai, S. Matsunaga, N. Kumagai, Acc. Chem. Res. 2009, 42, 1117.
- 2For selected recent examples, see:
- 2aB. M. Trost, J. Hitce, J. Am. Chem. Soc. 2009, 131, 4572;
- 2bC. Mazet, E. N. Jacobsen, Angew. Chem. 2008, 120, 1786;
10.1002/ange.200704461 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 1762;
- 2cS. Handa, V. Gnanadesikan, S. Matsunaga, M. Shibasaki, J. Am. Chem. Soc. 2010, 132, 4925;
- 2dK. Endo, M. Ogawa, T. Shibata, Angew. Chem. 2010, 122, 2460;
10.1002/ange.200906839 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 2410.
- 3For reviews on bifunctional organocatalysts, see:
- 3aM. S. Taylor, E. N. Jacobsen, Angew. Chem. 2006, 118, 1550; Angew. Chem. Int. Ed. 2006, 45, 1520;
- 3bS. J. Connon, Chem. Commun. 2008, 2499;
- 3cA. Dondoni, A. Massi, Angew. Chem. 2008, 120, 4716;
10.1002/ange.200704684 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 4638;
- 3dC. Palomo, M. Oiarbide, R. López, Chem. Soc. Rev. 2009, 38, 632.
- 4For selected examples of bifunctional metal/tethered amine catalysts, see:
- 4aE. F. DiMauro, M. C. Kozlowski, J. Am. Chem. Soc. 2002, 124, 12668;
- 4bS. France, M. H. Shah, A. Weatherwax, H. Wack, J. P. Roth, T. Lectka, J. Am. Chem. Soc. 2005, 127, 1206;
- 4cY.-M. Lin, J. Boucau, Z. Li, V. Casarotto, J. Lin, A. N. Nguyen, J. Ehrmantraut, Org. Lett. 2007, 9, 567;
- 4dF. Yang, D. Zhao, J. Lan, P. Xi, L. Yang, S. Xiang, J. You, Angew. Chem. 2008, 120, 5728; Angew. Chem. Int. Ed. 2008, 47, 5646;
- 4eT. Kull, J. Cabrera, R. Peters, Chem. Eur. J. 2010, 16, 9132.
- 5For a recent example of cooperative catalysis that comprises a Lewis acid and an amine–urea organocatalyst, see: T. Yang, A. Ferrali, F. Sladojevich, L. Campbell, D. J. Dixon, J. Am. Chem. Soc. 2009, 131, 9140.
- 6For recent reviews, see:
- 6aC. Palomo, M. Oiarbide, A. Laso, Eur. J. Org. Chem. 2007, 2561; for selected examples, see:
- 6bH. Sasai, T. Suzuki, S. Arai, T. Arai, M. Shibasaki, J. Am. Chem. Soc. 1992, 114, 4418;
- 6cC. Christensen, K. Juhl, R. G. Hazell, K. A. Jørgensen, J. Org. Chem. 2002, 67, 4875;
- 6dB. M. Trost, V. S. C. Yeh, Angew. Chem. 2002, 114, 889;
10.1002/1521-3757(20020301)114:5<889::AID-ANGE889>3.0.CO;2-8 Google ScholarAngew. Chem. Int. Ed. 2002, 41, 861;10.1002/1521-3773(20020301)41:5<861::AID-ANIE861>3.0.CO;2-V CAS PubMed Web of Science® Google Scholar
- 6eD. A. Evans, D. Seidel, M. Rueping, H. W. Lam, J. T. Shaw, C. W. Downey, J. Am. Chem. Soc. 2003, 125, 12692;
- 6fC. Palomo, M. Oiarbide, A. Laso, Angew. Chem. 2005, 117, 3949;
10.1002/ange.200463075 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 3881.
- 7For syn-selective asymmetric Henry reactions, see:
- 7aH. Sasai, T. Tokunaga, S. Watanabe, T. Suzuki, N. Itoh, M. Shibasaki, J. Org. Chem. 1995, 60, 7388;
- 7bY. Sohtome, Y. Hashimoto, K. Nagasawa, Eur. J. Org. Chem. 2006, 2894;
- 7cY. Sohtome, Y. Kato, S. Handa, N. Aoyama, K. Nagawa, S. Matsunaga, M. Shibasaki, Org. Lett. 2008, 10, 2231;
- 7dL. Cheng, J. Dong, J. You, G. Gao, J. Lan, Chem. Eur. J. 2010, 16, 6761;
- 7eT. Arai, R. Takashita, Y. Endo, M. Watanabe, A. Yanagisawa, J. Org. Chem. 2008, 73, 4903;
- 7fY. Zhou, J. Dong, F. Zhang, Y. Gong, J. Org. Chem. 2011, 76, 588.
- 8
- 8aD. Seebach, A. K. Beck, F. Lehr, T. Weller, E. Colvin, Angew. Chem. 1981, 93, 422; Angew. Chem. Int. Ed. Engl. 1981, 20, 397; for anti-selective asymmetric Henry reactions using silyl nitronates, see:
- 8bT. Risgaard, K. V. Gothelf, K. A. Jørgensen, Org. Biomol. Chem. 2003, 1, 153;
- 8cT. Ooi, K. Doda, K. Maruoka, J. Am. Chem. Soc. 2003, 125, 2054; for anti-selective Henry reactions using nitroalkanes directly, see:
- 8dD. Uraguchi, S. Sakaki, T. Ooi, J. Am. Chem. Soc. 2007, 129, 12392;
- 8eD. Uraguchi, S. Nakamura, T. Ooi, Angew. Chem. 2010, 122, 7724;
10.1002/ange.201004072 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 7562;
- 8fS. Handa, K. Nagawa, Y. Sohtome, S. Matsunaga, M. Shibasaki, Angew. Chem. 2008, 120, 3274;
10.1002/ange.200705617 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 3230;
- 8gT. Nitabaru, A. Nojiri, M. Kobayashi, N. Kumagai, M. Shibasaki, J. Am. Chem. Soc. 2009, 131, 13860;
- 8hG. Blay, L. R. Domingo, V. Hernández-Olmos, J. R. Pedro, Chem. Eur. J. 2008, 14, 4725;
- 8iG. Blay, V. Hernández-Olmos, J. R. Pedro, Org. Lett. 2010, 12, 3058.
- 9aK. Lang, J. Park, S. Hong, J. Org. Chem. 2010, 75, 6424;
- 9bJ. Park, K. Lang, K. A. Abboud, S. Hong, J. Am. Chem. Soc. 2008, 130, 16484;
- 9cJ. Park, K. Lang, K. A. Abboud, S. Hong, Chem. Eur. J. 2011, 17, 2236.
- 10For [(salen)metal]-catalyzed Henry reactions, see:
- 10aY. Kogami, T. Nakajima, T. Ikeno, T. Yamada, Synthesis 2004, 1947;
- 10bR. Kowalczyk, Ł. Sidorowicz, J. Skarżewski, Tetrahedron: Asymmetry 2007, 18, 2581;
- 10cR. Kowalczyk, P. Kwiatkowski, J. Skarżewski, J. Jurczak, J. Org. Chem. 2009, 74, 753;
- 10dA. Zulauf, M. Mellah, E. Schulz, J. Org. Chem. 2009, 74, 2242; also see ref. [9b].
- 11See the Supporting Information for details.
- 12To the best of our knowledge, there is only one report that shows significant anti selectivity for 2-alkoxy-containing benzaldehydes. Pedro and co-workers reported the Cu-catalyzed anti-selective Henry reaction between 2 a and nitroethane (3 a) to afford 4 aa in 95 % yield, anti:syn=82:18, and 95 %/94 % ee (anti/syn). See Ref. [8 h].
- 13
- 13aP. N. Patil, A. Tye, J. B. LaPidus, J. Pharmacol. Exp. Ther. 1967, 156, 445;
- 13bR. Baltzly, N. B. Mehta, J. Med. Chem. 1968, 11, 833;
- 13cR. M. DeMarinis, W. M. Bryan, D. H. Shah, J. P. Hieble, R. G. Pendleton, J. Med. Chem. 1981, 24, 1432;
- 13dO. M. Jones, J. M. Thompson, A. F. Brading, N. J. M. Mortensen, Br. J. Surg. 2003, 90, 872.
- 14M. Fujita, T. Hiyama, J. Org. Chem. 1988, 53, 5415.
- 15For a recent example showing the synergy between a chiral Co catalyst and a thiourea additive, see: H. Y. Kim, K. Oh, Org. Lett. 2011, 13, 1306.
- 16In contrast, urea additives decreased the rate of the [(salen)CoIII]-catalyzed hydrolytic kinetic resolution of epoxides. See Ref. [9 c].
- 17T. Satyanarayana, S. Abraham, H. B. Kagan, Angew. Chem. 2009, 121, 464;
10.1002/ange.200705241 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 456.
- 18Increased Lewis acidity of CoIII versus CoII might be a plausible reason for this mechanism change.
- 19First-order kinetics are observed for both bisurea-containing CoII catalyst 1 and CoIII catalyst 1⋅OBzF (see the Supporting Information for details). However, first-order kinetics alone cannot distinguish between the two mechanisms (Scheme 1 a versus b), as the first-order kinetics is expected from the covalently tethered bimetallic catalysts or strongly self-associating systems. For relevant discussions, see:
- 19aJ. M. Ready, E. N. Jacobsen, J. Am. Chem. Soc. 2001, 123, 2687;
- 19bD. G. Blackmond, Adv. Synth. Catal. 2002, 344, 156;
- 19cRef [9 c].
- 20The interaction between (thio)ureas and neutral nitro compounds is generally weak, see:
- 20aT. R. Kelly, M. H. Kim, J. Am. Chem. Soc. 1994, 116, 7072;
- 20bJ. Bu, N. D. Lilienthal, J. E. Woods, C. E. Nohrden, K. T. Hoang, D. Truong, D. K. Smith, J. Am. Chem. Soc. 2005, 127, 6423.
- 21Hamilton determined the Ka (120 M−1) between Bu4N+NO2CHCH3− and 1,3-dimethylthiourea in [D6]-DMSO. It was found that the negative charge on the nitronate is crucial for a strong association with thiourea, see: B. R. Linton, M. S. Goodman, A. D. Hamilton, Chem. Eur. J. 2000, 6, 2449.
10.1002/1521-3765(20000703)6:13<2449::AID-CHEM2449>3.0.CO;2-9 CAS PubMed Web of Science® Google Scholar
- 22For anion recognition by (thio)urea, see: C. Caltagirone, P. A. Gale, Chem. Soc. Rev. 2009, 38, 520.
- 23For reviews on H-bond-donor catalysis, see:
- 23aA. G. Doyle, E. N. Jacobsen, Chem. Rev. 2007, 107, 5713;
- 23bZ. Zhang, P. R. Schreiner, Chem. Soc. Rev. 2009, 38, 1187.
- 24For reviews on catalysis involving (thio)urea/nitro(nate)interactions, see:
- 24aY. Takemoto, Chem. Pharm. Bull. 2010, 58, 593; for selected examples, see:
- 24bT. P. Yoon, E. N. Jacobsen, Angew. Chem. 2005, 117, 470; Angew. Chem. Int. Ed. 2005, 44, 466;
- 24cT. Okino, Y. Hoashi, T. Furukawa, X. Xu, Y. Takemoto, J. Am. Chem. Soc. 2005, 127, 119;
- 24dC. Rabalakos, W. D. Wulff, J. Am. Chem. Soc. 2008, 130, 13524;
- 24eW. J. Nodes, D. R. Nutt, A. M. Chippindale, A. J. A. Cobb, J. Am. Chem. Soc. 2009, 131, 16016.