Asymmetric Synthesis of Chiral 1,3-Diaminopropanols: Bisoxazolidine-Catalyzed CC Bond Formation with α-Keto Amides†
Hanhui Xu
Department of Chemistry, Georgetown University, 37th and O Streets, Washington, DC 20057 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Christian Wolf
Department of Chemistry, Georgetown University, 37th and O Streets, Washington, DC 20057 (USA)
Department of Chemistry, Georgetown University, 37th and O Streets, Washington, DC 20057 (USA)Search for more papers by this authorHanhui Xu
Department of Chemistry, Georgetown University, 37th and O Streets, Washington, DC 20057 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Christian Wolf
Department of Chemistry, Georgetown University, 37th and O Streets, Washington, DC 20057 (USA)
Department of Chemistry, Georgetown University, 37th and O Streets, Washington, DC 20057 (USA)Search for more papers by this authorFunding from the National Science Foundation (CHE-0848301) is gratefully acknowledged.
Graphical Abstract
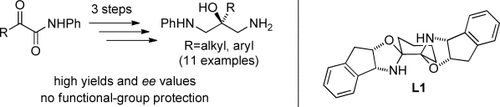
Three high-yielding steps lead to the formation of chiral 1,3-diaminopropanols from aliphatic and aromatic α-keto amides. In this approach, a nitroaldol reaction, which is catalyzed by Cu(SO2CF3)2 and the bisoxazolidine ligand L1, is followed by two mild reduction reactions (see scheme). Laborious protection and deprotection steps can be avoided by using this method.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201105778_sm_miscellaneous_information.pdf2.3 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aG.-Q. Lin, Y.-M. Li, A. S. C. Chan, Principles and Applications of Asymmetric Synthesis, Wiley-VCH, New York, 2001;
10.1002/0471220426 Google Scholar
- 1bC. Wolf, Dynamic Stereochemistry of Chiral Compounds, RSC Publishing, Cambridge, 2008.
- 2
- 2aS. J. Brickner, M. R. Barbachyn, D. K. Hutchinson, P. R. Manninen, J. Med. Chem. 2008, 51, 1981;
- 2bF. Russo, F. Wangsell, J. Savmarker, M. Jacobsson, M. Larhed, Tetrahedron 2009, 65, 10047;
- 2cA. Kamal, R. V. C. R. Shetti, S. Azeeza, P. Swapna, M. N. A. Khan, I. A. Khan, S. Sharma, S. T. Abdullah, Eur. J. Med. Chem. 2011, 46, 893.
- 3
- 3aJ. Sasai, T. Suzuki, S. Arai, T. Arai, M. Shibasaki, J. Am. Chem. Soc. 1992, 114, 4418;
- 3bJ. Sasai, T. Suzuki, N. Itoh, M. Shibasaki, Tetrahedron Lett. 1993, 34, 851–854;
- 3cH. Sasai, N. Zuzuki, K. Itoh, T. Tanaka, K. Date, K. Okamura, M. Shibasaki, J. Am. Chem. Soc. 1993, 115, 10372–10373;
- 3dY. Sohtome, Y. Kato, S. Handa, N. Aoyama, K. Nagawa, S. Matsunaga, M. Shibasaki, Org. Lett. 2008, 10, 2231–2234;
- 3eT. Nitabaru, N. Kumagai, M. Shibasaki, Tetrahedron Lett. 2008, 49, 272–276.
- 4
- 4aB. M. Trost, V. S. C. Yeh, Angew. Chem. 2002, 114, 889;
10.1002/1521-3757(20020301)114:5<889::AID-ANGE889>3.0.CO;2-8 Google ScholarAngew. Chem. Int. Ed. 2002, 41, 861;10.1002/1521-3773(20020301)41:5<861::AID-ANIE861>3.0.CO;2-V CAS PubMed Web of Science® Google Scholar
- 4bC. Christensen, K. Juhl, R. G. Hazell, K. A. Jørgensen, J. Org. Chem. 2002, 67, 4875;
- 4cT. Ooi, K. Doda, K. Maruoka, J. Am. Chem. Soc. 2003, 125, 2054;
- 4dD. A. Evans, D. Seidel, M. Rueping, H. W. Lam, J. T. Shaw, C. W. Downey, J. Am. Chem. Soc. 2003, 125, 12692;
- 4eC. Palomo, M. Oiarbide, A. Mielgo, Angew. Chem. 2004, 116, 5558;
10.1002/ange.200460506 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 5442;
- 4fC. Palomo, M. Oiarbide, A. Laso, Angew. Chem. 2005, 117, 3949;
10.1002/ange.200463075 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 3881;
- 4gM. Bandini, F. Piccinelli, S. Tommasi, A. Umani-Ronchi, C. Ventrici, Chem. Commun. 2007, 616;
- 4hB. M. Choudary, K. V. S. Ranganath, U. Pal, M. L. Kantam, B. Sreedhar, J. Am. Chem. Soc. 2005, 127, 13167;
- 4iT. Arai, M. Watanabe, A. Fujiwara, N. Yokoyama, A. Yanagisawa, Angew. Chem. 2006, 118, 6124;
10.1002/ange.200602255 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 5978;
- 4jH. Y. Kim, K. Oh, Org. Lett. 2009, 11, 5682;
- 4kM. Steurer, C. Bolm, J. Org. Chem. 2010, 75, 3301;
- 4lK. Lang, J. Park, S. Hong, J. Org. Chem. 2010, 75, 6424;
- 4mD. Uraguchi, S. Nakamura, T. Ooi, Angew. Chem. 2010, 122, 7724;
10.1002/ange.201004072 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 7562;
- 4nV. J. Mayani, S. H. R. Abdi, R. I. Kureshy, N.-u. H. Khan, A. Das, H. C. Bajaj, J. Org. Chem. 2010, 75, 6191;
- 4oW. Jin, X. Li, B. Wan, J. Org. Chem. 2011, 76, 484.
- 5
- 5aC. Christensen, K. Juhl, K. A. Jørgensen, Chem. Commun. 2001, 2222;
- 5bD.-M. Du, S.-F. Lu, T. Fang, J. Xu, J. Org. Chem. 2005, 70, 3712;
- 5cH. Li, B. Wang, L. Deng, J. Am. Chem. Soc. 2006, 128, 732;
- 5dF. Tur, J. M. Saa, Org. Lett. 2007, 9, 5079;
- 5eB. Qin, X. Xiao, X. Liu, J. Huang, Y. Wen, X. Feng, J. Org. Chem. 2007, 72, 9323;
- 5fA. Bulut, A. Aslant, O. Dogan, J. Org. Chem. 2008, 73, 7373;
- 5gM. Bandini, R. Sinisi, A. Umani-Ronchi, Chem. Commun. 2008, 4360;
- 5hG. Blay, V. Hernandez-Olmos, J. R. Pedro, Chem. Eur. J. 2011, 17, 3768.
- 6For an example of asymmetric catalysis with an α-formyl amide, see: D. A. Evans, Y. Aye, J. Am. Chem. Soc. 2006, 128, 11034. During the completion of this manuscript, the asymmetric intramolecular arylation of α-keto amides was reported: L. Yin, M. Kanai, M. Shibasaki, Angew. Chem. 2011, 123, 7762; Angew. Chem. Int. Ed. 2011, 50, 7620. Racemic nitroaldol products of keto amides have been obtained by using catalytic amounts of diethyl- or triethylamine:
- 6aW. R. Conn, H. G. Lindwall, J. Am. Chem. Soc. 1936, 58, 1236;
- 6bY.-J. Wang, Z.-X. Shen, Y.-W. Zhang, Chin. J. Org. Chem. 2006, 26, 1291.
- 7Nitroaldol products are known to easily decompose and racemize under basic and acidic conditions or upon heating.
- 8For desymmetrization of 1,3-propanediols and 1,2,3-propanetriols, see:
- 8aB. Jung, S. H. Kang, Proc. Natl. Acad. Sci. USA 2007, 104, 1471;
- 8bJ. Y. Lee, Y. S. You, S. H. Kang, J. Am. Chem. Soc. 2011, 133, 1772.
- 9
- 9aC. A. Caputo, N. D. Jones, Dalton Trans. 2007, 4627;
- 9bC. Wolf, H. Xu, Chem. Commun. 2011, 47, 3339.
- 10
- 10aC. Wolf, S. Liu, J. Am. Chem. Soc. 2006, 128, 10996;
- 10bS. Liu, C. Wolf, Org. Lett. 2007, 9, 2965;
- 10cS. Liu, C. Wolf, Org. Lett. 2008, 10, 1831;
- 10dK. Yearick Spangler, C. Wolf, Org. Lett. 2009, 11, 4724;
- 10eH. Xu, C. Wolf, Chem. Commun. 2010, 46, 8026;
- 10fH. Xu, C. Wolf, Synlett 2010, 2765;
- 10gC. Wolf, P. Zhang, Adv. Synth. Catal. 2011, 353, 760. This ligand is commercially available at Strem (cat. no. 07-0488), US Patent, 11/737,371, 2007.
- 11We found that in most cases double condensation to generate a bisoxazolidine structure does not occur with a wide range of amino alcohols and diketones. In contrast, only one carbonyl group reacts even under harsh conditions to give a monooxazolidine, or other by-products are formed.
- 12
- 12aC. M. Bellucci, A. Bergamini, P. G. Cozzi, A. Papa, E. Tagliavini, A. Umani-Ronchi, Tetrahedron: Asymmetry 1997, 8, 895;
- 12bS. Higashijima, H. Itoh, Y. Senda, S. Nakano, Tetrahedron: Asymmetry 1997, 8, 3107.
- 13
- 13aF. Fringuelli, R. Girotti, F. Pizzo, L. Vaccaro, Org. Lett. 2006, 8, 2487;
- 13bK. Tanaka, T. Kobayashi, H. Mori, S. Katsumura, J. Org. Chem. 2004, 69, 5906.
- 14The absolute configuration of the nitroaldol products was confirmed by NMR analysis with a chiral solvating agent (see the Supporting Information).
- 15The nitroaldol reaction with N-propyl and N-benzyl 2-oxo-2-phenylacetamide gave the product in 92–95 % yield and 26–30 % ee.
- 16
- 16aA. A. Núñez Magro, G. R. Eastham, D. J. Cole-Hamilton, Chem. Commun. 2007, 3154;
- 16bS. Das, D. Addis, S. Zhou, K. Junge, M. Beller, J. Am. Chem. Soc. 2010, 132, 1770.
- 17Formation of the tBoc derivative of 25 and HPLC analysis on a chiral stationary phase (Chiralpak AD) showed that the ee value was slightly reduced from 90 % to 86 % after the two reduction steps (see the Supporting Information).