ReO2Cl(S2O7), a Molecular Disulfate†
Dipl.-Chem. Ulf Betke
Carl von Ossietzky University of Oldenburg, Institute for Pure and Applied Chemistry, Carl-von-Ossietzky Strasse 9–11, 26129 Oldenburg (Germany)
Search for more papers by this authorWilke Dononelli
Carl von Ossietzky University of Oldenburg, Institute for Pure and Applied Chemistry, Carl-von-Ossietzky Strasse 9–11, 26129 Oldenburg (Germany)
Search for more papers by this authorProf. Dr. Thorsten Klüner
Carl von Ossietzky University of Oldenburg, Institute for Pure and Applied Chemistry, Carl-von-Ossietzky Strasse 9–11, 26129 Oldenburg (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Mathias S. Wickleder
Carl von Ossietzky University of Oldenburg, Institute for Pure and Applied Chemistry, Carl-von-Ossietzky Strasse 9–11, 26129 Oldenburg (Germany)
Carl von Ossietzky University of Oldenburg, Institute for Pure and Applied Chemistry, Carl-von-Ossietzky Strasse 9–11, 26129 Oldenburg (Germany)Search for more papers by this authorDipl.-Chem. Ulf Betke
Carl von Ossietzky University of Oldenburg, Institute for Pure and Applied Chemistry, Carl-von-Ossietzky Strasse 9–11, 26129 Oldenburg (Germany)
Search for more papers by this authorWilke Dononelli
Carl von Ossietzky University of Oldenburg, Institute for Pure and Applied Chemistry, Carl-von-Ossietzky Strasse 9–11, 26129 Oldenburg (Germany)
Search for more papers by this authorProf. Dr. Thorsten Klüner
Carl von Ossietzky University of Oldenburg, Institute for Pure and Applied Chemistry, Carl-von-Ossietzky Strasse 9–11, 26129 Oldenburg (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Mathias S. Wickleder
Carl von Ossietzky University of Oldenburg, Institute for Pure and Applied Chemistry, Carl-von-Ossietzky Strasse 9–11, 26129 Oldenburg (Germany)
Carl von Ossietzky University of Oldenburg, Institute for Pure and Applied Chemistry, Carl-von-Ossietzky Strasse 9–11, 26129 Oldenburg (Germany)Search for more papers by this authorFinancial support of the Deutsche Forschungsgemeinschaft, the Fonds der Chemischen Industrie, and the Heinz-Neumüller Stiftung is gratefully acknowledged. We thank Wolfgang Saak for the collection of the X-ray data. We are also thankful to Regina Stötzel and to Prof. Claudia Wickleder (both University of Siegen) for Raman measurements.
Graphical Abstract
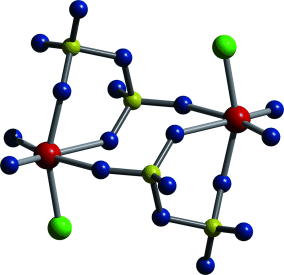
A molecular metal disulfate, Re2O4Cl2(S2O7)2 forms in the reaction of ReCl5 and oleum (65 % SO3) under harsh conditions. The first of its kind, this unique ReVII compound contains two ReO2Cl fragments linked by two disulfate groups, leading to Ci-symmetric molecules (see picture; Re red, O blue, S yellow, Cl green).
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201105457_sm_miscellaneous_information.pdf727 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1K. Ståhl, T. Balic-Zunic, F. da Silva, K. M. Eriksen, R. W. Berg, R. Fehrmann, J. Solid State Chem. 2005, 178, 1697–1704.
- 2H. Lynton, M. R. Truter, J. Chem. Soc. 1960, 5112–5118.
- 3K. Ståhl, R. W. Berg, K. M. Eriksen, R. Fehrmann, Acta Crystallogr. Sect. B 2009, 65, 551–557.
- 4I. D. Brown, D. B. Crump, R. J. Gillespie, Inorg. Chem. 1971, 10, 2319–2323.
- 5J. Douglade, R. Mercier, Acta Crystallogr. Sect. B 1979, 35, 1062–1067.
- 6F. W. B. Einstein, A. C. Willis, Acta Crystallogr. Sect. B 1981, 37, 218–220.
- 7M. A. Simonov, S. V. Shkovrov, S. I. Troyanov, Sov. Phys. Crystallogr. 1988, 33, 297.
- 8M. Jansen, R. Müller, Z. Anorg. Allg. Chem. 1997, 623, 1055–1060.
- 9W. Hönle, Z. Kristallogr. 1991, 196, 279–288.
- 10A. Simon, A. Pischtschan, Z. Anorg. Allg. Chem. 1966, 344, 10–17.
- 11R. Appel, R. Frechen, Z. Anorg. Allg. Chem. 1977, 428, 125–128.
- 12U. Betke, M. S. Wickleder, Inorg. Chem. 2011, 50, 858–872.
- 13U. Betke, K. Neuschulz, M. S. Wickleder, Chem. Eur. J. 2011, 17, 8538–8541.
- 14U. Betke, M. S. Wickleder, Eur. J. Inorg. Chem. 2011, 4400–4413.
- 15M. Hołyńska, T. Lis, Acta Crystallogr. Sect. C 2008, 64, i 18–i20.
- 16J. Supeł, K. Seppelt, Z. Anorg. Allg. Chem. 2007, 633, 227–230.
- 17B. J. Coe, S. J. Glenwright, Coord. Chem. Rev. 2000, 203, 5–80.
- 18P. Metrangolo, H. Neukirch, T. Pilati, G. Resnati, Acc. Chem. Res. 2005, 38, 386–395.
- 19L. Brammer, G. Mínguez Espallargas, S. Libri, CrystEngComm 2008, 10, 1712–1727.
- 20J. Supeł, K. Seppelt, Angew. Chem. 2006, 118, 4791–4793;
10.1002/ange.200504468 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 4675–4677.
- 21U. Betke, M. S. Wickleder, Z. Anorg. Allg. Chem. 2011, DOI: .
- 22W. Noh, G. S. Girolami, Dalton Trans. 2007, 674–679.
- 23Gaussian 03, Revision C.02, M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery, Jr., T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez, and J. A. Pople, Gaussian, Inc., Wallingford CT, 2004.
- 24Y. Tantirungrotechai, K. Phanasant, S. Roddecha, P. Surawatanawong, V. Sutthikhum, J. Limtrakul, J. Mol. Struct. THEOCHEM. 2006, 760, 189–192.