Mechanistic Studies of an IspH-Catalyzed Reaction: Implications for Substrate Binding and Protonation in the Biosynthesis of Isoprenoids†
Wei-chen Chang
Division of Medicinal Chemistry, College of Pharmacy and Department of Chemistry and Biochemistry, University of Texas at Austin, Austin, TX 78712 (USA)
These authors contributed equally to this work.
Search for more papers by this authorDr. Youli Xiao
Department of Chemistry, Boston University, Boston, MA 02215 (USA)
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Prof. Dr. Hung-wen Liu
Division of Medicinal Chemistry, College of Pharmacy and Department of Chemistry and Biochemistry, University of Texas at Austin, Austin, TX 78712 (USA)
Hung-wen Liu, Division of Medicinal Chemistry, College of Pharmacy and Department of Chemistry and Biochemistry, University of Texas at Austin, Austin, TX 78712 (USA)
Pinghua Liu, Department of Chemistry, Boston University, Boston, MA 02215 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Pinghua Liu
Department of Chemistry, Boston University, Boston, MA 02215 (USA)
Hung-wen Liu, Division of Medicinal Chemistry, College of Pharmacy and Department of Chemistry and Biochemistry, University of Texas at Austin, Austin, TX 78712 (USA)
Pinghua Liu, Department of Chemistry, Boston University, Boston, MA 02215 (USA)
Search for more papers by this authorWei-chen Chang
Division of Medicinal Chemistry, College of Pharmacy and Department of Chemistry and Biochemistry, University of Texas at Austin, Austin, TX 78712 (USA)
These authors contributed equally to this work.
Search for more papers by this authorDr. Youli Xiao
Department of Chemistry, Boston University, Boston, MA 02215 (USA)
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Prof. Dr. Hung-wen Liu
Division of Medicinal Chemistry, College of Pharmacy and Department of Chemistry and Biochemistry, University of Texas at Austin, Austin, TX 78712 (USA)
Hung-wen Liu, Division of Medicinal Chemistry, College of Pharmacy and Department of Chemistry and Biochemistry, University of Texas at Austin, Austin, TX 78712 (USA)
Pinghua Liu, Department of Chemistry, Boston University, Boston, MA 02215 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Pinghua Liu
Department of Chemistry, Boston University, Boston, MA 02215 (USA)
Hung-wen Liu, Division of Medicinal Chemistry, College of Pharmacy and Department of Chemistry and Biochemistry, University of Texas at Austin, Austin, TX 78712 (USA)
Pinghua Liu, Department of Chemistry, Boston University, Boston, MA 02215 (USA)
Search for more papers by this authorFinancial support for this work was provided in part by grants from the National Science Foundation (CAREER, CHE-0748504 to P.L.), the National Institutes of Health (GM 093903 to P.L.), and the Welch Foundation (F-1511 to H.-w.L.).
Graphical Abstract
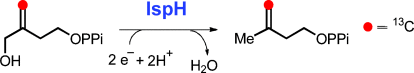
Chosen handle: Mechanistic studies of the IspH-catalyzed reductive dehydroxylation of 4-hydroxy-3-methyl-2-(E)-1-diphosphate (HMBPP) to isopentenyl diphosphate and dimethylallyl diphosphate suggest that both the 4-OH group and the double bond of HMBPP may contribute to the formation of substrate–IspH complex. Labeling studies now show that the 4-hydroxy group of the substrate plays the dominant role in positioning the substrate in the enzyme active site.
References
- 1
- 1aJ. C. Sacchettini, C. D. Poulter, Science 1997, 277, 1788–1789;
- 1bW. Eisenreich, A. Bacher, D. Arigoni, F. Rohdich, Cell. Mol. Life Sci. 2004, 61, 1401–1426;
- 1cT. Dairi, T. Kuzuyama, M. Nishiyama, I. Fujii, Nat. Prod. Rep. 2011, 28, 1054–1086.
- 2F. Bouvier, A. Rahier, B. Camara, Prog. Lipid Res. 2005, 44, 357–429.
- 3
- 3aF. Khachik, G. R. Beecher, J. C. Smith, Jr., J. Cell. Biochem. 1995, 22, 236–246;
- 3bB. Demmig-Adams, W. W. Adams, Science 2002, 298, 2149–2153.
- 4L. Ruzicka, Experientia 1994, 50, 395–405.
- 5
- 5aD. J. McGarvey, R. Croteau, Plant Cell 1995, 7, 1015–1026;
- 5bT. J. Bach, Lipids 1995, 30, 191–202;
- 5cK. Bloch, Steroids 1992, 57, 378–383;
- 5dT. Kuzuyama, H. Hemmi, S. Takahashi in Comprehensive Natural Products II, Chemistry and Biology, Vol. I (Eds.: ), Elsevier, Amsterdam, 2010, pp. 493–516.
10.1016/B978-008045382-8.00014-9 Google Scholar
- 6
- 6aM. Rohmer, Prog. Drug Res. 1998, 50, 135–154;
- 6bM. Rohmer, M. Knani, P. Simonin, B. Sutter, H. Sahm, Biochem. J. 1993, 295(Pt 2), 517–524;
- 6cM. Rohmer in Comprehensive Natural Products II, Chemistry and Biology, Vol. I (Eds.: ), Elsevier, Amsterdam, 2010, pp. 517–556.
10.1016/B978-008045382-8.00702-4 Google Scholar
- 7
- 7aP. Adam, S. Hecht, W. Eisenreich, J. Kaiser, T. Gräwert, D. Arigoni, A. Bacher, F. Rohdich, Proc. Natl. Acad. Sci. USA 2002, 99, 12108–12113;
- 7bF. Rohdich, S. Hecht, K. Gartner, P. Adam, C. Krieger, S. Amslinger, D. Arigoni, A. Bacher, W. Eisenreich, Proc. Natl. Acad. Sci. USA 2002, 99, 1158–1163;
- 7cB. Altincicek, E. C. Duin, A. Reichenberg, R. Hedderich, A. K. Kollas, M. Hintz, S. Wagner, J. Wiesner, E. Beck, H. Jomaa, FEBS Lett. 2002, 532, 437–440;
- 7dT. Gräwert, J. Kaiser, F. Zepeck, R. Laupitz, S. Hecht, S. Amslinger, N. Schramek, E. Schleicher, S. Weber, M. Haslbeck, J. Buchner, C. Rieder, D. Arigoni, A. Bacher, W. Eisenreich, F. Rohdich, J. Am. Chem. Soc. 2004, 126, 12847–12855;
- 7eM. Wolff, M. Seemann, C. Grosdemange-Billiard, D. Tritsch, N. Campos, M. Rodríguez-Concepción, A. Boronat, M. Rohmer, Tetrahedron Lett. 2002, 43, 2555–2559;
- 7fI. Rekittke, J. Wiesner, R. Rohrich, U. Demmer, E. Warkentin, W. Y. Xu, K. Troschke, M. Hintz, J. H. No, E. C. Duin, E. Oldfield, H. Jomaa, U. Ermler, J. Am. Chem. Soc. 2008, 130, 17206–17207;
- 7gK. Wang, W. Wang, J. H. No, Y. Zhang, E. Oldfield, J. Am. Chem. Soc. 2010, 132, 6719–6727.
- 8
- 8aT. Gräwert, I. Span, A. Bacher, M. Groll, Angew. Chem. 2010, 122, 8984–8991;
10.1002/ange.201000833 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 8802–8809;
- 8bY. Xiao, L. Chu, Y. Sanakis, P. Liu, J. Am. Chem. Soc. 2009, 131, 9931–9933;
- 8cW. Wang, K. Wang, Y. L. Liu, J. H. No, J. Li, M. J. Nilges, E. Oldfield, Proc. Natl. Acad. Sci. USA 2010, 107, 4522–4527.
- 9
- 9aF. Rohdich, F. Zepeck, P. Adam, S. Hecht, J. Kaiser, R. Laupitz, T. Gräwert, S. Amslinger, W. Eisenreich, A. Bacher, D. Arigoni, Proc. Natl. Acad. Sci. USA 2003, 100, 1586–1591;
- 9bY. Xiao, Z. K. Zhao, P. Liu, J. Am. Chem. Soc. 2008, 130, 2164–2165.
- 10
- 10aT. Gräwert, F. Rohdich, I. Span, A. Bacher, W. Eisenreich, J. Eppinger, M. Groll, Angew. Chem. 2009, 121, 5867–5870;
10.1002/ange.200900548 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 5756–5759;
- 10bT. Gräwert, I. Span, W. Eisenreich, F. Rohdich, J. Eppinger, A. Bacher, M. Groll, Proc. Natl. Acad. Sci. USA 2010, 107, 1077–1081.
- 11Y. Xiao, P. Liu, Angew. Chem. 2008, 120, 9868–9871; Angew. Chem. Int. Ed. 2008, 47, 9722–9725.
- 12
- 12aE. Oldfield, Acc. Chem. Res. 2010, 43, 1216–1226;
- 12bM. Rodríguez-Concepción, Curr. Pharm. Des. 2004, 10, 2391–2400.
- 13M. Seemann, K. Janthawornpong, J. Schweizer, L. H. Böttger, A. Janoschka, A. Ahrens-Botzong, M. N. Tambou, O. Rotthaus, A. X. Trautwein, M. Rohmer, V. Schunemann, J. Am. Chem. Soc. 2009, 131, 13184–13185.
- 14R. Laupitz, T. Gräwert, C. Rieder, F. Zepeck, A. Bacher, D. Arigoni, F. Rohdich, W. Eisenreich, Chem. Biodiversity 2004, 1, 1367–1376.
- 15M. Shanmugam, B. Zhang, R. L. McNaughton, R. A. Kinney, R. Hille, B. M. Hoffman, J. Am. Chem. Soc. 2010, 132, 14015–14017.