Ruthenium Bis(σ-BH) Aminoborane Complexes from Dehydrogenation of Amine–Boranes: Trapping of H2BNH2†
We thank the CNRS and the ANR (programme blanc ANR HyBoCat 2009) for support.
Graphical Abstract
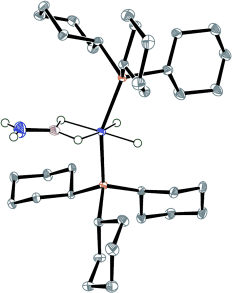
One small step for ammonia–borane: The simplest elementary aminoborane compound H2BNH2, which results from dehydrogenation of ammonia–borane, has been trapped by a ruthenium complex fragment leading to the isolation of a bis(σ-BH) aminoborane complex. The analogous H2BNHMe and H2BNMe2 complexes were also prepared. (Picture: ruthenium complex; Ru purple, P orange, N blue, B brown, H white.)
Ammonia–borane has attracted considerable interest recently as a potential hydrogen source and storage material owing to its high hydrogen storage capacity.1 Homogeneous transition-metal-catalyzed dehydrogenation of ammonia–boranes leads to dihydrogen release; however, reversible storage from the resulting polymeric materials remains a major problem.2 The nature of the transition metal complex used to catalyze the dehydrogenation of the amine–borane family H3BNR3−nHn (n=1–3) (I) has a direct impact both on kinetics and dehydrocoupling routes.3 However, the successive elementary steps leading to dehydrocoupling are still not well understood, and the question of the BH/NH bond activation mechanism is a very active area of research.4
BH bond activation of a tertiary amine–borane adduct is achieved in the η1-H3BNR3 Shimoi-type complexes in the case of chromium, tungsten, and manganese (Scheme 1).5 In such a case, the substitution pattern at the nitrogen atom prevents further dihydrogen release. Thus, these complexes can be regarded as early intermediates in amine–borane BH activation processes.6 Starting from precursors I that are likely to undergo dehydrogenation, known complexes retaining a BN unit are restricted to two cationic rhodium examples, as reported by Weller et al. They are amine–borane adducts with η1- and/or η2-H3BNRR′H bonding modes (R,R′=H, Me; Scheme 1).7, 8
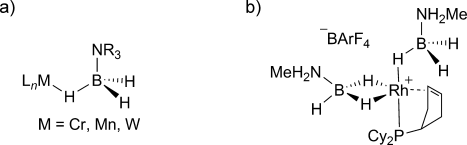
Representative examples of a) η1-H3BNR3 Shimoi and b) η1- and η2-bis(H3BNH2Me) Weller complexes.
With this work in mind, and following our recent findings on the isolation of bis(σ-BH) complexes from the reaction of dihydrogenoboranes with the bis(dihydrogen) complex [RuH2(η2-H2)2(PCy3)2],9, 10 we herein report the synthesis of the first “true” bis (σ-BH) aminoborane ruthenium complexes.11 Stabilization of the simplest aminoborane units H2BNMen−2Hn (n=1–2) results from the stoichiometric dehydrogenation of the amine–borane precursors by the ruthenium complex.
Room-temperature reaction of [RuH2(η2-H2)2(PCy3)2] in toluene with the amine–boranes 1 a–c proceeds with gas evolution. After workup, the resulting solids, which analyzed as [RuH2(η2:η2-H2B-NR1R2)(PCy3)2] (2), were isolated as white powders (2 a: 77 %, 2 b: 60 %, 2 c: 64 %; Scheme 2) and fully characterized by NMR and X-ray diffraction crystallography for 2 a.
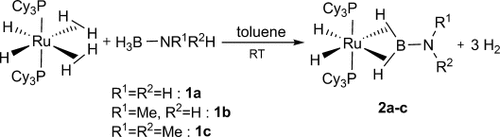
Synthesis of 2 by dehydrogenation of amine–boranes 1 a–c.
As a representative example, we will describe the NMR spectroscopic properties of 2 a, but similar features are observed for compounds 2 b–c and are reminiscent of the spectroscopic data reported for the ruthenium complexes [RuH2(η2:η2-H2B-R)(PCy3)2] (R=tBu, Mes).9 The 1H NMR spectrum of 2 a in the hydride region in C6D6 at 298 K exhibits a broad singlet at δ=−6.80 ppm and a more shielded triplet (JPH=24.8 Hz) in a 1:1 integration ratio at δ=−11.85 ppm. The triplet collapsed into a singlet upon phosphorus decoupling, whereas the singlet sharpened upon boron decoupling. A 1D TOCSY 1H{11B} experiment with a selective excitation at δ=−6.80 ppm showed correlations with the triplet and a deshielded singlet at δ=2.34 ppm, which were assigned to the NH2 protons (see the Supporting Information, Figure S1). The 31P{1H} NMR spectrum shows a sharp singlet at δ=77.43 ppm, and a broad signal is observed in the 11B{1H} NMR spectrum at δ=46 ppm. This 11B signal is highly shifted from the resonance of the starting ammonia–borane (δ=−21.6 ppm)12 and slightly downfield from those of free monomeric aminoboranes (H2BNR2 δ=35–36 ppm).13
The X-ray structure of 2 a was determined at 110 K (Figure 1 and Table 1). The quality of the measurement allows us to be confident on the location of the hydrogen atoms around the metal.14 The ruthenium atom is in a pseudo-octahedral environment with the phosphines in axial positions. The four hydrogen atoms (H1–4) surrounding the metal, the boron, the nitrogen, and the NH2 hydrogen atoms are all located in the equatorial plane. The RuB distance (1.956(2) Å) is shorter than the sum of the covalent radii (2.09 Å) and similar to the distances previously reported for the bis(σ-BH) borane ruthenium complexes.9, 15 The most striking features are the shortening of the BN distance (1.396(3) Å) with respect to that of ammonia–borane (1.58(2) Å as determined by neutron diffraction),16 and the increase of the BH bond distances (1.25(2) and 1.22(3) Å) compared to 1.15(3) and 1.18(3) Å in H3BNH3.16
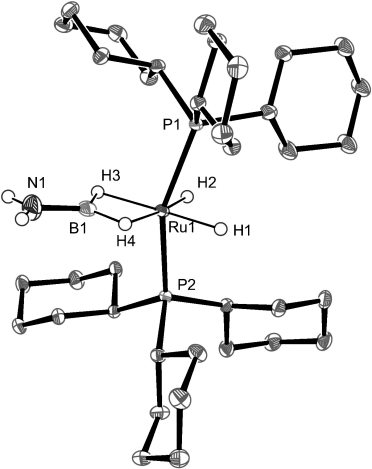
X-ray crystal structure of [RuH2(η2:η2-H2BNH2)(PCy3)2] (2 a). Ellipsoids set at 50 % probability; phosphine hydrogen atoms omitted for clarity.
|
2 a |
3 a |
H2BNH2 |
|
|---|---|---|---|
|
Ru–B |
1.956(2) |
1.960 |
|
|
Ru–P1 |
2.3215(5) |
2.307 |
|
|
Ru–P2 |
2.3131(5) |
2.311 |
|
|
B–N |
1.396(3) |
1.403 |
1.390 |
|
Ru–H1 |
1.54(2) |
1.612 |
|
|
Ru–H2 |
1.55(2) |
1.612 |
|
|
Ru–H3 |
1.71(2) |
1.811 |
|
|
Ru–H4 |
1.73(3) |
1.811 |
|
|
B–H3 |
1.25(2) |
1.323 |
1.198 |
|
B–H4 |
1.22(3) |
1.323 |
1.198 |
|
P1-Ru-P2 |
155.066(19) |
156.76 |
|
|
H3-B-H6 |
120.3(16) |
127.01 |
121.9 |
|
P1-Ru-B |
102.90(7) |
100.60 |
|
|
P2-Ru-B |
102.03(7) |
102.64 |
|
|
Ru-B-N |
179.4(2) |
178.85 |
- [a] Distances in Å and angles in degrees.
DFT(B3PW91) calculations on the model compound [Ru(H)2(H2BNH2)(PMe3)2] (3) were carried out to characterize the mode of coordination of H2BNH2. Four different isomers were located on the potential energy surface (see the Supporting Information, Figure S3). The most stable isomer is the bis(σ-BH) adduct 3 a, the model for 2 a. The π adducts of H2BNH2, 3 c and 3 d, are significantly less stable (ΔE=19.0 kcal mol−1, 3 c; ΔE=14.3 kcal mol−1, 3 d). The fourth isomer, 3 b, features both coordination of NH2 and of a σ-BH bond and lies 11.4 kcal mol−1 above 3 a. Therefore, the bis(σ-BH) adduct 3 a corresponds to the most stable mode of coordination of aminoborane to [Ru(H2)(PMe3)2]. Moreover, the calculated energy variations for the reaction [Ru(H)2(H2)2(PMe3)2]+H3BNH3→2 a+3H2 are indicative of both an exothermic (ΔH=−2.5 kcal mol−1) and an exergonic (ΔG=−15.7 kcal mol−1) transformation. The σ-BH bonds thus compete efficiently with H2 for coordination to ruthenium and dissociation of three dihydrogen molecules entropically drives the reaction toward facile formation of 3 a.
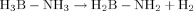 ((1))
((1))In conclusion, compounds 2 a–c are the first examples of “true” bis(σ-BH) monomeric aminoborane complexes. They result from the simple metal-assisted dehydrogenation of amine–boranes and can be regarded as intermediates in the dehydrogenation process generally leading to polyaminoboranes. Remarkably, the bis-σ coordination mode enables the stabilization of monomeric aminoboranes in the coordination sphere of a metal, including the simplest and elusive prototypical H2BNH2 elementary unit.
Experimental Section
2 a–c: Two to four equivalents of amine–boranes 1 a–c were added to a toluene solution of [RuH2(η2-H2)2(PCy3)2] (typically 300 mg) at room temperature, and the reaction was monitored by 31P NMR spectroscopy until consumption of all the starting ruthenium complex was observed. After removal of the solvent, the residue was dried under vacuum, dissolved in pentane, and filtered. After evaporation of the solvent, compounds 2 a–c were isolated as air-sensitive white solids (2 a: 77 %, 2 b: 60 %, 2 c: 64 %). In the case of 1 a, the resulting toluene solution was filtered after reaction before evaporation of the solvent.
Selected data for 2 a–c: 2 a: 31P{1H} NMR (C6D6, 298 K, 161.975 MHz): δ=77.44 ppm. 11B{1H} NMR (C6D6, 298 K, 128.377 MHz): δ=46 ppm (br). 1H NMR (C6D6, 298 K, 400.130 MHz): δ=1.2–2.5 (m, 68 H, NH2+Cy), −6.80 (br s, 2 H, BH2), −11.85 ppm (t, 2 H, JPH=24.8 Hz, RuH2). IR (Nujol):  =3484 and 3394 (s, NHs,as), 1971 and 1923 (m br, RuH), 1833 and 1785 (m br, RuHB), 1583 cm−1 (s, NHbend). Elemental analysis (%) calcd for C36H72BNP2Ru: C 62.41; H 10.48; N 2.02; found: C 62.80; H 10.34; N 2.05.
=3484 and 3394 (s, NHs,as), 1971 and 1923 (m br, RuH), 1833 and 1785 (m br, RuHB), 1583 cm−1 (s, NHbend). Elemental analysis (%) calcd for C36H72BNP2Ru: C 62.41; H 10.48; N 2.02; found: C 62.80; H 10.34; N 2.05.
2 b: 31P{1H} NMR (C6D6, 298 K, 202.537 MHz): δ=78.50 ppm. 11B{1H} NMR (C6D6, 298 K, 160.525 MHz): δ=45.7 ppm. 1H NMR (C6D6, 298 K, 500.330 MHz): δ=2.675 (d, 3 H, 3JHH=5 Hz, CH3), 2.48 (br, 1 H, NH), 1.2–2.4 (m, 66 H, Cy), −6.80 (br s, 2 H, BH2), −11.99 ppm (t, 2 H, JPH=24.4 Hz, RuH2). Elemental analysis (%) calcd for C37H74BNP2Ru: C 62.87; H 10.55; N 1.98; found: C 63.04; H 9.49; N 2.11.
2 c: 31P{1H} NMR (C6D6, 298 K, 161.975 MHz): δ=79.34 ppm. 11B{1H} NMR (C6D6, 298 K, 128.377 MHz): δ=47.7 ppm (br). 1H NMR (C6D6, 298 K, 400.127 MHz): δ=2.80 (s, 6 H, NMe2), 1.2–2.5 (m, 66 H, Cy), −6.89 (br s, 2 H, BH2), −12.17(t, 2 H, JPH=24.6 Hz, RuH2). Elemental analysis (%) calcd for C38H76BNP2Ru: C 63.32; H 10.63; N 1.94; found: C 63.69; H 9.63; N 1.75.
CCDC 751581 (2 a) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.