A Catalytic Asymmetric 6 π Electrocyclization: Enantioselective Synthesis of 2-Pyrazolines†
Steffen Müller
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany), Fax: (+49) 208-306-2999
Search for more papers by this authorBenjamin List Prof. Dr.
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany), Fax: (+49) 208-306-2999
Search for more papers by this authorSteffen Müller
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany), Fax: (+49) 208-306-2999
Search for more papers by this authorBenjamin List Prof. Dr.
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany), Fax: (+49) 208-306-2999
Search for more papers by this authorWe thank our X-ray department and D. Bock for crystal structure analysis and H. van Thienen and Dr. V. Wakchaure for technical assistance. Generous support by the Max Planck Society, the Deutsche Forschungsgemeinschaft (SPP 1179, Organokatalyse), and the Fonds der Chemischen Industrie (Award to B.L. and Fellwoship to S.M.) is gratefully acknowledged.
Graphical Abstract
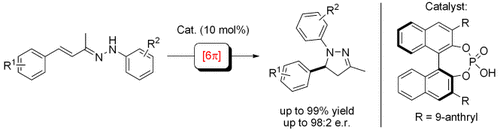
A closer look at α,β-unsaturated hydrazonium ions reveals that they are isoelectronic to the pentadienyl anion, making them suitable substrates to undergo 6π electrocyclizations. The enantioselective catalysis of this transformation is achieved for the first time by asymmetric Brønsted acid catalysis.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200905035_sm_miscellaneous_information.pdf2.6 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aH. B. Kagan, O. Riant, Chem. Rev. 1992, 92, 1007–1019;
- 1bK. V. Gothelf, K. A. Jørgensen, Chem. Rev. 1998, 98, 863–909;
- 1cK. A. Jørgensen, Angew. Chem. 2000, 112, 3702–3733;
10.1002/1521-3757(20001016)112:20<3702::AID-ANGE3702>3.0.CO;2-R Google ScholarAngew. Chem. Int. Ed. 2000, 39, 3558–3588;10.1002/1521-3773(20001016)39:20<3558::AID-ANIE3558>3.0.CO;2-I CAS PubMed Web of Science® Google Scholar
- 1dE. J. Corey, Angew. Chem. 2002, 114, 1724–1741;
10.1002/1521-3757(20020517)114:10<1724::AID-ANGE1724>3.0.CO;2-Q Google ScholarAngew. Chem. Int. Ed. 2002, 41, 1650–1667;10.1002/1521-3773(20020517)41:10<1650::AID-ANIE1650>3.0.CO;2-B CAS PubMed Web of Science® Google Scholar
- 1eL. M. Stanley, M. P. Sibi, Chem. Rev. 2008, 108, 2887–2902;
- 1f Cycloaddition Reactions in Organic Synthesis (Eds.: ), Wiley-VCH, Weinheim, 2002.
- 2For reviews, see:
- 2a The Claisen Rearrangement (Eds.: ), Wiley-VCH, Weinheim, 2007;
- 2bM. Hiersemann, L. Abraham, Eur. J. Org. Chem. 2002, 1461–1471;
- 2cU. Nubbemeyer, Synthesis 2003, 961–1008;
- 2dA. M. Martín Castro, Chem. Rev. 2004, 104, 2939–3002. For selected recent examples, see:
- 2eL. Abraham, R. Czerwonka, M. Hiersemann, Angew. Chem. 2001, 113, 4835–4837;
10.1002/1521-3757(20011217)113:24<4835::AID-ANGE4835>3.0.CO;2-D Google ScholarAngew. Chem. Int. Ed. 2001, 40, 4700–4703;10.1002/1521-3773(20011217)40:24<4700::AID-ANIE4700>3.0.CO;2-6 CAS PubMed Web of Science® Google Scholar
- 2fC. E. Anderson, L. E. Overman, J. Am. Chem. Soc. 2003, 125, 12412–12413;
- 2gC. Uyeda, E. N. Jacobsen, J. Am. Chem. Soc. 2008, 130, 9228–9229;
- 2hM. Rueping, A. P. Antonchick, Angew. Chem. 2008, 120, 10244–10247; Angew. Chem. Int. Ed. 2008, 47, 10090–10093.
- 3For noncatalytic, diastereoselective 6π electrocyclizations, see for example:
- 3aS. J. Veenstra, W. N. Speckamp, J. Chem. Soc. Chem. Commun. 1982, 369–370;
- 3bK. Tanaka, S. Katsumura, J. Am. Chem. Soc. 2002, 124, 9660–9661;
- 3cT. Kobayashi, F. Hasegawa, K. Tanaka, S. Katsumura, Org. Lett. 2006, 8, 3813–3816;
- 3dC. L. Benson, F. G. West, Org. Lett. 2007, 9, 2545–2548;
- 3efor applications in biomimetic synthesis, see: C. M. Beaudry, J. P. Malerich, D. Trauner, Chem. Rev. 2005, 105, 4757–4778.
- 4
- 4aG. Liang, S. N. Gradl, D. Trauner, Org. Lett. 2003, 5, 4931–4934;
- 4bV. K. Aggarwal, A. J. Belfield, Org. Lett. 2003, 5, 5075–5078;
- 4cG. Liang, D. Trauner, J. Am. Chem. Soc. 2004, 126, 9544–9545.
- 5For an organocatalytic variant, see:
- 5aM. Rueping, W. Ieawsuwan, A. P. Antonchick, B. J. Nachtsheim, Angew. Chem. 2007, 119, 2143–2146;
10.1002/ange.200604809 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 2097–2100;
- 5bM. Rueping, W. Ieawsuwan, Adv. Synth. Catal. 2009, 351, 78–84.
- 6
- 6aL. M. Bishop, J. E. Barbarow, R. G. Bergman, D. Trauner, Angew. Chem. 2008, 120, 8220–8223; Angew. Chem. Int. Ed. 2008, 47, 8100–8103;
- 6bD. J. Tantillo, Angew. Chem. 2009, 121, 33–34;
10.1002/ange.200804908 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 31–32.
- 7E. Fischer, O. Knoevenagel, Justus Liebigs Ann. Chem. 1887, 239, 194–206.
10.1002/jlac.18872390205 Google Scholar
- 8R. Huisgen, Angew. Chem. 1980, 92, 979–1005; Angew. Chem. Int. Ed. Engl. 1980, 19, 947–973.
- 9
- 9aE. Palaska, D. Erol, R. Demirdamar, Eur. J. Med. Chem. 1996, 31, 43–47;
- 9bJ. H. M. Lange, H. K. A. C. Coolen, H. H. van Stuivenberg, J. A. R. Dijksman, A. H. J. Herremans, E. Ronken, H. G. Keizer, K. Tipker, A. C. McCreary, W. Veerman, H. C. Wals, B. Stork, P. C. Verveer, A. P. den Hartog, N. M. J. de Jong, T. J. P. Adolfs, J. Hoogendoorn, C. G. Kruse, J. Med. Chem. 2004, 47, 627–643;
- 9cF. Chimenti, A. Bolasco, F. Manna, D. Secci, P. Chimenti, O. Befani, P. Turini, V. Giovannini, B. Mondovì, R. Cirilli, F. La Torre, J. Med. Chem. 2004, 47, 2071–2074;
- 9dY. Rajendra Prasad, A. Lakshmana Rao, L. Prasoona, K. Murali, P. Ravi Kumar, Bioorg. Med. Chem. Lett. 2005, 15, 5030–5034;
- 9eN. Gökhan-Keleçi, S. Koyunoğlu, S. Yabanoğlu, K. Yelekçi, Ö. Özgen, G. Uçar, K. Erol, E. Kendi, A. Yeşilada, Bioorg. Med. Chem. 2009, 17, 675–689;
- 9fC. D. Cox, M. J. Breslin, B. J. Mariano, P. J. Coleman, C. A. Buser, E. S. Walsh, K. Hamilton, H. E. Huber, N. E. Kohl, M. Torrent, Y. Yan, L. C. Kuo, G. D. Hartman, Bioorg. Med. Chem. Lett. 2005, 15, 2041–2045;
- 9gC. D. Cox, M. Torrent, M. J. Breslin, B. J. Mariano, D. B. Whitman, P. J. Coleman, C. A. Buser, E. S. Walsh, K. Hamilton, M. D. Schaber, R. B. Lobell, W. Tao, V. J. South, N. E. Kohl, Y. Yan, L. C. Kuo, T. Prueksaritanont, D. E. Slaughter, C. Li, E. Mahan, B. Lu, G. D. Hartman, Bioorg. Med. Chem. Lett. 2006, 16, 3175–3179;
- 9hF. Manna, F. Chimenti, A. Bolasco, M. L. Cenicola, M. D′Amico, C. Parrillo, F. Rossi, E. Marmo, Eur. J. Med. Chem. 1992, 27, 633–639;
- 9iF. Chimenti, B. Bizzarri, F. Manna, A. Bolasco, D. Secci, P. Chimenti, A. Granese, D. Rivanera, D. Lilli, M. M. Scaltrito, M. I. Brenciaglia, Bioorg. Med. Chem. Lett. 2005, 15, 603–607;
- 9jJ. R. Goodell, F. Puig-Basagoiti, B. M. Forshey, P.-Y. Shi, D. M. Ferguson, J. Med. Chem. 2006, 49, 2127–2137;
- 9kX. Zhang, X. Li, G. F. Allan, T. Sbriscia, O. Linton, S. G. Lundeen, Z. Sui, J. Med. Chem. 2007, 50, 3857–3869;
- 9lM. E. Camacho, J. León, A. Entrena, G. Velasco, M. D. Carrión, G. Escames, A. Vivó, D. Acuña-Castroviejo, M. A. Gallo, A. Espinosa, J. Med. Chem. 2004, 47, 5641–5650.
- 10
- 10aE. Buchner, M. Fritsch, A. Papendieck, H. Witter, Justus Liebigs Ann. Chem. 1893, 273, 214–231;
10.1002/jlac.18932730207 Google Scholar
- 10bH. von Pechmann, Ber. Dtsch. Chem. Ges. 1894, 27, 1888–1891.
10.1002/cber.189402702141 Google Scholar
- 11For a review on the synthesis of pyrazolines, see: A. Léavai, J. Heterocycl. Chem. 2002, 39, 1–13.
- 12
- 12aS. Kanemasa, T. Kanai, J. Am. Chem. Soc. 2000, 122, 10710–10711;
- 12bR. Shintani, G. C. Fu, J. Am. Chem. Soc. 2003, 125, 10778–10779;
- 12cY. Yamashita, S. Kobayashi, J. Am. Chem. Soc. 2004, 126, 11279–11282;
- 12dT. Kano, T. Hashimoto, K. Maruoka, J. Am. Chem. Soc. 2006, 128, 2174–2175;
- 12eM. P. Sibi, L. M. Stanley, T. Soeta, Org. Lett. 2007, 9, 1553–1556;
- 12fL. Gao, G.-S. Hwang, M. Y. Lee, D. H. Ryu, Chem. Commun. 2009, 5460–5462.
- 13H. Yanagita, S. Kanemasa, Heterocycles 2007, 71, 699–709.
- 14For an organocatalytic but racemic version, see: O. Mahé, D. Frath, I. Dez, F. Marsais, V. Levacher, J.-F. Brière, Org. Biomol. Chem. 2009, 7, 3648–3651.
- 15At the same time, Sibi and Soeta reported a related asymmetric conjugate addition cyclization sequence of α,β-unsaturated imides and hydrazines: M. P. Sibi, T. Soeta, J. Am. Chem. Soc. 2007, 129, 4522–4523.
- 16H. Ferres, M. S. Hamdam, W. R. Jackson, J. Chem. Soc. B 1971, 1892–1898.
- 17
- 17aT. Akiyama, Chem. Rev. 2007, 107, 5744–5758;
- 17bM. Terada, Chem. Commun. 2008, 4097–4112.
- 18
- 18aR. Cuberes, J. Berrocal, M. Contijoch, J. Frigola, WO 99/62884, 1999;
- 18bC. Calvet, R. Cuberes, C. Pérez-Maseda, J. Frigola, Electrophoresis 2002, 23, 1702–1708.
10.1002/1522-2683(200206)23:11<1702::AID-ELPS1702>3.0.CO;2-# CAS PubMed Web of Science® Google Scholar
- 19
- 19aM. Behforouz, J. L. Bolan, M. S. Flynt, J. Org. Chem. 1985, 50, 1186–1189;
- 19bS. Faramarz Tayyari, J. L. Speakman, M. B. Arnold, W. Cai, M. Behforouz, J. Chem. Soc. Perkin Trans. 2 1998, 2195–2200;
- 19cS. Uchiyama, M. Ando, S. Aoyagi, J. Chromatogr. A 2003, 996, 95–102;
- 19dS. Uchiyama, E. Matsushima, S. Aoyagi, M. Ando, Anal. Chim. Acta 2004, 523, 157–163.
- 20After acceptance of this manuscript (received: September 8, 2009), another catalytic asymmetric 6π electrocyclization appeared: E. E. Maciver, S. Thompson, M. D. Smith, Angew. Chem. 2009, 121, 10164–10167; Angew. Chem. Int. Ed. 2009, 48, 9979–9982 (received: September 15, 2009).