Multimodal Magnetic-Resonance/Optical-Imaging Contrast Agent Sensitive to NADH†
Chuqiao Tu
Department of Biomedical Engineering, University of California, Davis, 1 Shields Avenue, Davis, CA 95616 (USA), Fax: (+1) 530-754-5739
Search for more papers by this authorRyan Nagao
Department of Biomedical Engineering, University of California, Davis, 1 Shields Avenue, Davis, CA 95616 (USA), Fax: (+1) 530-754-5739
Search for more papers by this authorAngelique Y. Louie Prof.
Department of Biomedical Engineering, University of California, Davis, 1 Shields Avenue, Davis, CA 95616 (USA), Fax: (+1) 530-754-5739
Search for more papers by this authorChuqiao Tu
Department of Biomedical Engineering, University of California, Davis, 1 Shields Avenue, Davis, CA 95616 (USA), Fax: (+1) 530-754-5739
Search for more papers by this authorRyan Nagao
Department of Biomedical Engineering, University of California, Davis, 1 Shields Avenue, Davis, CA 95616 (USA), Fax: (+1) 530-754-5739
Search for more papers by this authorAngelique Y. Louie Prof.
Department of Biomedical Engineering, University of California, Davis, 1 Shields Avenue, Davis, CA 95616 (USA), Fax: (+1) 530-754-5739
Search for more papers by this authorThis research is supported by the National Institute of Health (RO3 EY13941-01) and NMR award of the University of California, Davis. We thank Dr. Benjamin R. Jarrett for help with cell experiments.
Graphical Abstract
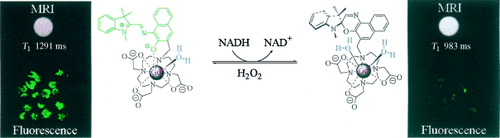
Agents get smart: A macrocycle-based gadolinium complex undergoes an isomerization in the presence of NADH, leading to an r1 relaxivity increase of 54 %, while the intense fluorescence disappears (see scheme). This agent could facilitate the use of magnetic resonance imaging (MRI) for probing tissue redox status.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200900984_sm_miscellaneous_information.pdf3 MB | miscellaneous information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1A. T. Hoye, J. E. Davoren, P. Wipf, M. P. Fink, V. E. Kagan, Acc. Chem. Res. 2008, 41, 87–97.
- 2
- 2aD. A. Mankoff, J. Nucl. Med. 2007, 48, 18N; D. A. Mankoff, J. Nucl. Med. 2007, 48, 21N;
- 2bQ. Z. Cao, W. B. Cai, G. Niu, L. N. He, X. Y. Chen, Clin. Cancer Res. 2008, 14, 6137–6145.
- 3C. Westbrook, C. K. Roth, J. Talbot, MRI in Practice, 3rd ed., Blackwell, Malden, 2005.
- 4
- 4aV. Jacques, J. F. Desreux, Top. Curr. Chem. 2002, 221, 123–164;
- 4bJ. Ramos, M. Sirisawad, R. Miller, L. Naumovski, Mol. Cancer Ther. 2006, 5, 1176–1182;
- 4cF. Hyodo, K. H. Chuang, A. G. Goloshevsky, A. Sulima, G. L. Griffiths, J. B. Mitchell, A. P. Koretsky, M. C. Krishna, J. Cereb. Blood Flow Metab. 2008, 28, 1165–1174.
- 5F. Hyodo, B. P. Soule, K. I. Matsumoto, S. Matusmoto, J. A. Cook, E. Hyodo, A. L. Sowers, M. C. Krishna, J. B. Mitchell, J. Pharm. Pharmacol. 2008, 60, 1049–1060.
- 6
- 6aJ. Zhi, R. Baba, K. Hashimoto, A. Fujishima, J. Photochem. Photobiol. A 1995, 92, 91–97;
- 6bK. Kimura, T. Yamashita, M. Yokoyama, Chem. Soc. Perkin Trans. 2 1992, 613–619.
- 7C. Q. Tu, A. Y. Louie, Chem. Commun. 2007, 1331–1333.
- 8
- 8aN. Y. C. Chu, Can. J. Chem. 1983, 61, 300–305;
- 8bW. Yuan, L. Sun, H. Tang, Y. Wen, G. Jiang, W. Huang, L. Jiang, Y. Song, H. Tian, D. Zhu, Adv. Mater. 2005, 17, 156–160.
- 9
- 9aV. Adam-Vizi, C. Chinopoulos, Trends Pharmacol. Sci. 2006, 27, 639–645;
- 9bL. M. Forsyth, H. G. Preuss, A. L. MacDowell, L. Chiazze, G. D. Birkmayer, J. A. Bellanti, Ann. Allergy Asthma Immunol. 1999, 82, 185–191;
- 9cB. B. Seo, E. Nakamaru-Ogiso, T. R. Flotte, T. Yagi, A. Matsuno-Yagi, Mol. Therapy 2002, 6, 336–341;
- 9dK. Nadlinger, J. Birkmayer, F. Gebauer, R. Kunze, Neuroimmunomodulation 2001, 9, 203–208.
- 10
- 10aW. Denk, J. H. Strikler, W. W. Webb, Science 1990, 248, 73–76;
- 10bH. D. Vishwasrao, A. A. Heikal, K. A. Kasischke, W. W. Webb, J. Biol. Chem. 2005, 280, 25119–25126;
- 10cG. H. Patterson, S. M. Knobel, P. Arkhammar, O. Thastrup, D. W. Piston, Proc. Natl. Acad. Sci. USA 2000, 97, 5203–5207;
- 10dR. Freeman, R. Gill, I. Shweky, M. Kotler, U. Banin, I. Willner, Angew. Chem. 2009, 121, 315–319; Angew. Chem. Int. Ed. 2009, 48, 309–313.
- 11
- 11aA. Samat, V. Lokshin, K. Chamontin, D. Levi, G. Pepe, R. Guglielmetti, Tetrahedron 2001, 57, 7349–7359;
- 11bA. Dadabhoy, S. Faulkner, P. G. Sammes, J. Chem. Soc. Perkin Trans. 2 2002, 348–357.
- 12Z. B. Zhang, C. R. Zhang, M. G. Fan, W. P. Yan, Dyes Pigm. 2008, 77, 469–473.
- 13
- 13aK. Kimura, T. Yamashita, M. Yokoyama, J. Chem. Soc. Perkin Trans. 2 1992, 613–619;
- 13bS. H. Liu, X. Y. Wu, C. T. Wu, Acta Chim. Sin. 1999, 57, 1167.
- 14D. Magde, G. E. Rojas, P. Seybold, Photochem. Photobiol. 1999, 70, 737–744.
- 15K. Djanashvili, J. A. Peters, Contrast Media Mol. Imaging 2007, 2, 67–71.
- 16M. C. Alpoim, A. M. Urbano, C. F. G. C. Geraldes, J. P. Peters, J. Chem. Soc., Dalton Trans. 1992, 403–407.
- 17P. Caravan, J. J. Ellison, T. J. McMurry, R. B. Lauffer, Chem. Rev. 1999, 99, 2293.
- 18J. O’Brien, I. Wilson, T. Orton, F. Pognan, Eur. J. Biochem. 2000, 267, 5421–5426.
- 19M. Koval, K. Preiter, C. Adles, P. D. Stahl, T. H. Steinberg, Exp. Cell. Res. 1998, 242, 265–273.
- 20
- 20aL. K. Klaidman, A. C. Leung, J. D. Adams, Anal. Biochem. 1995, 228, 312–317;
- 20bD. K. Merrill, R. W. Guynn, Brain Res. 1981, 221, 307–318.
- 21C. Q. Tu, E. A. Osborne, A. Y. Louie, Tetrahedron 2009, 65, 1241-1246.