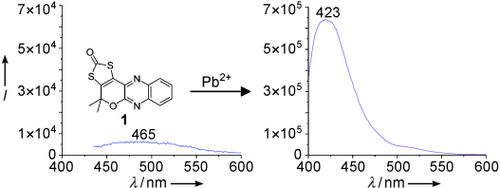
Development of a Fluorescent Pb2+ Sensor†
We thank the National Institutes of Health for partial financial support of this research. Lauren Marbella is a John V. Crable fellow. We thank Drs. Bernd Hammann, Mizanur Rahman, and Mitchell Johnson for experimental assistance and helpful discussions.