Phosphinoborane and Sulfidoborohydride as Chelating Ligands in Polyhydride Ruthenium Complexes: Agostic σ-Borane versus Dihydroborate Coordination†
Yann Gloaguen
CNRS, LCC (Laboratoire de Chimie de Coordination), 205 route de Narbonne, 31077 Toulouse (France) http://www.lcc-toulouse.fr/lcc/spip.php?article433
Université de Toulouse, UPS, INPT, 31077 Toulouse (France)
Search for more papers by this authorGilles Alcaraz Dr.
CNRS, LCC (Laboratoire de Chimie de Coordination), 205 route de Narbonne, 31077 Toulouse (France) http://www.lcc-toulouse.fr/lcc/spip.php?article433
Université de Toulouse, UPS, INPT, 31077 Toulouse (France)
Search for more papers by this authorAnne-Frédérique Pécharman
CNRS, LCC (Laboratoire de Chimie de Coordination), 205 route de Narbonne, 31077 Toulouse (France) http://www.lcc-toulouse.fr/lcc/spip.php?article433
Université de Toulouse, UPS, INPT, 31077 Toulouse (France)
Search for more papers by this authorEric Clot Dr.
Université Montpellier 2, Institut Charles Gerhardt, CNRS 5253, cc 1501, Place Eugène Bataillon, 34095 Montpellier (France)
Search for more papers by this authorLaure Vendier Dr.
CNRS, LCC (Laboratoire de Chimie de Coordination), 205 route de Narbonne, 31077 Toulouse (France) http://www.lcc-toulouse.fr/lcc/spip.php?article433
Université de Toulouse, UPS, INPT, 31077 Toulouse (France)
Search for more papers by this authorSylviane Sabo-Etienne Dr.
CNRS, LCC (Laboratoire de Chimie de Coordination), 205 route de Narbonne, 31077 Toulouse (France) http://www.lcc-toulouse.fr/lcc/spip.php?article433
Université de Toulouse, UPS, INPT, 31077 Toulouse (France)
Search for more papers by this authorYann Gloaguen
CNRS, LCC (Laboratoire de Chimie de Coordination), 205 route de Narbonne, 31077 Toulouse (France) http://www.lcc-toulouse.fr/lcc/spip.php?article433
Université de Toulouse, UPS, INPT, 31077 Toulouse (France)
Search for more papers by this authorGilles Alcaraz Dr.
CNRS, LCC (Laboratoire de Chimie de Coordination), 205 route de Narbonne, 31077 Toulouse (France) http://www.lcc-toulouse.fr/lcc/spip.php?article433
Université de Toulouse, UPS, INPT, 31077 Toulouse (France)
Search for more papers by this authorAnne-Frédérique Pécharman
CNRS, LCC (Laboratoire de Chimie de Coordination), 205 route de Narbonne, 31077 Toulouse (France) http://www.lcc-toulouse.fr/lcc/spip.php?article433
Université de Toulouse, UPS, INPT, 31077 Toulouse (France)
Search for more papers by this authorEric Clot Dr.
Université Montpellier 2, Institut Charles Gerhardt, CNRS 5253, cc 1501, Place Eugène Bataillon, 34095 Montpellier (France)
Search for more papers by this authorLaure Vendier Dr.
CNRS, LCC (Laboratoire de Chimie de Coordination), 205 route de Narbonne, 31077 Toulouse (France) http://www.lcc-toulouse.fr/lcc/spip.php?article433
Université de Toulouse, UPS, INPT, 31077 Toulouse (France)
Search for more papers by this authorSylviane Sabo-Etienne Dr.
CNRS, LCC (Laboratoire de Chimie de Coordination), 205 route de Narbonne, 31077 Toulouse (France) http://www.lcc-toulouse.fr/lcc/spip.php?article433
Université de Toulouse, UPS, INPT, 31077 Toulouse (France)
Search for more papers by this authorWe thank the CNRS and the ANR (programme blanc ANR-06-BLAN-0060-01) for support (Y.G., G.A., L.V., S.S.E.)
Graphical Abstract
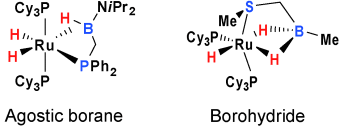
A question of coordination mode: Two new borane compounds are prepared. They act as bifunctional ligands as illustrated by their reaction with ruthenium polyhydrides which leads to the formation of two complexes (see scheme) displaying either a δ-agostic interaction of a η2-BH bond involving a trivalent boron atom or a dihydroborate ligation.
Abstract
A question of coordination mode: Two new borane compounds are prepared. They act as bifunctional ligands as illustrated by their reaction with ruthenium polyhydrides which leads to the formation of two complexes (see scheme) displaying either a δ-agostic interaction of a η2-BH bond involving a trivalent boron atom or a dihydroborate ligation.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200806178_sm_miscellaneous_information.pdf207.5 KB | miscellaneous information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1G. J. Kubas, Chem. Rev. 2007, 107, 4152–4205.
- 2G. J. Kubas, Metal Dihydrogen and σ-Bond Complexes, Kluwer Academic/Plenum Publishers, New York, 2001.
10.1007/b113929 Google Scholar
- 3J. Y. Corey, J. Braddock-Wilking, Chem. Rev. 1999, 99, 175–292.
- 4S. Lachaize, S. Sabo-Etienne, Eur. J. Inorg. Chem. 2006, 2115–2127.
- 5G. Alcaraz, S. Sabo-Etienne, Coord. Chem. Rev. 2008, 252, 2395–2409.
- 6J. F. Hartwig, C. N. Muhoro, X. He, O. Eisenstein, R. Bosque, F. Maseras, J. Am. Chem. Soc. 1996, 118, 10936–10937.
- 7T. J. Hebden, M. C. Denney, V. Pons, P. M. B. Piccoli, T. F. Koetzle, A. J. Schultz, W. Kaminsky, K. I. Goldberg, D. M. Heinekey, J. Am. Chem. Soc. 2008, 130, 10812–10820.
- 8T. B. Marder, Z. Lin, Contemporary Metal Boron Chemistry I. Borylenes, Boryls, Borane σ-complexes, and borohydrides, Vol. 130, Springer, Berlin, 2008.
- 9K. K. Pandey, Coord. Chem. Rev. 2009, 253, 37–55.
- 10J. F. Hartwig, K. S. Cook, M. Hapke, C. D. Incarvito, Y. Fan, C. E. Webster, M. B. Hall, J. Am. Chem. Soc. 2005, 127, 2538–2552.
- 11R. N. Perutz, S. Sabo-Etienne, Angew. Chem. 2007, 119, 2630–2645;
10.1002/ange.200603224 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 2578–2592.
- 12M. C. Denney, V. Pons, T. J. Hebden, D. M. Heinekey, K. I. Goldberg, J. Am. Chem. Soc. 2006, 128, 12048–12049.
- 13T. M. Douglas, A. B. Chaplin, A. S. Weller, J. Am. Chem. Soc. 2008, 130, 14432–14433.
- 14N. Blaquiere, S. Diallo-Garcia, S. I. Gorelsky, D. A. Black, K. Fagnou, J. Am. Chem. Soc. 2008, 130, 14034–14035.
- 15B. L. Dietrich, K. I. Goldberg, D. M. Heinekey, T. Autrey, J. C. Linehan, Inorg. Chem. 2008, 47, 8583–8585.
- 16F. H. Stephens, V. Pons, T. R. Baker, Dalton Trans. 2007, 2613–2626.
- 17G. Alcaraz, E. Clot, U. Helmstedt, L. Vendier, S. Sabo-Etienne, J. Am. Chem. Soc. 2007, 129, 8704–8705.
- 18G. Alcaraz, U. Helmstedt, E. Clot, L. Vendier, S. Sabo-Etienne, J. Am. Chem. Soc. 2008, 130, 12878–12879.
- 19M. Brookhart, M. L. H. Green, L.-L. Wong, Prog. Inorg. Chem. 1988, 36, 1–124.
- 20True σ-complexes can be defined as a complex in which the σ-ligand is only coordinated to the metal center through the σ-bond, whereas in an agostic species, the ligand is bound to the metal by an additional group (either L or X). We define true σ-boranes as complexes containing neutral HBR2 ligands.
- 21F.-G. Fontaine, J. Boudreau, M.-H. Thibault, Eur. J. Inorg. Chem. 2008, 5439–5454.
- 22H. Braunschweig, R. Dirk, U. Englert, Z. Anorg. Allg. Chem. 1997, 623, 1093–1097.
- 23H. Braunschweig, R. D. Dewhurst, T. Herbst, K. Radacki, Angew. Chem. 2008, 120, 6067–6069; Angew. Chem. Int. Ed. 2008, 47, 5978–5980.
- 24V. Montiel-Palma, M. Lumbierres, B. Donnadieu, S. Sabo-Etienne, B. Chaudret, J. Am. Chem. Soc. 2002, 124, 5624–5625.
- 25S. Lachaize, K. Essalah, V. Montiel-Palma, L. Vendier, B. Chaudret, J. C. Barthelat, S. Sabo-Etienne, Organometallics 2005, 24, 2935–2943.
- 26R. T. Baker, J. C. Calabrese, S. A. Westcott, T. B. Marder, J. Am. Chem. Soc. 1995, 117, 8777–8784.
- 27H. Braunschweig, R. Dirk, B. Ganter, J. Organomet. Chem. 1997, 545–546, 257–266.
- 28The shortest bond (1.313 Å) is observed trans to the strongest ligand, the hydride. The σ(BH) to σ*(RuH) 2nd-order perturbation energy amounts to 288 kJ mol−1, a value significantly lower than the σ(BH) to σ*(RuP) 2nd-order perturbation energy of 442.7 kJ mol−1 for the donation of the BH bond trans to the phosphine. As a matter of fact, the larger donation from the BH bond trans to P explains the longer bond observed for BH (1.366 Å).
- 29M. Svensson, S. Humbel, R. D. J. Fröse, T. Matsubara, S. Sieber, K. Morokuma, J. Phys. Chem. 1996, 100, 19357–19363.
- 30A. D. Becke, J. Chem. Phys. 1993, 98, 5648–5652.
- 31J. P. Perdew, Y. Wang, Phys. Rev. B 1992, 45, 13244–13249.
- 32D. Andrae, U. Häussermann, M. Dolg, H. Stoll, H. Preuss, Theor. Chim. Acta 1990, 77, 123–141.
- 33A. Bergner, M. Dolg, W. Kuchle, H. Stoll, H. Preuss, Mol. Phys. 1993, 80, 1431–1441.
- 34A. W. Ehlers, M. Böhme, S. Dapprich, A. Gobbi, A. Höllwarth, V. Jonas, K. F. Köhler, R. Stegmann, A. Veldkamp, G. Frenking, Chem. Phys. Lett. 1993, 208, 111–114.
- 35A. Höllwarth, H. Böhme, S. Dapprich, A. W. Ehlers, A. Gobbi, V. Jonas, K. F. Köhler, R. Stegmann, A. Veldkamp, G. Frenking, Chem. Phys. Lett. 1993, 208, 237–240.
- 36P. C. Hariharan, J. A. Pople, Theor. Chim. Acta 1973, 28, 213–222.
- 37P. J. Hay, W. R. Wadt, J. Chem. Phys. 1985, 82, 270–283.
- 38W. R. Wadt, P. J. Hay, J. Chem. Phys. 1985, 82, 284–298.
- 39R. Ditchfield, W. J. Hehre, J. A. Pople, J. Chem. Phys. 1971, 54, 724–728.
- 40A. E. Reed, L. A. Curtiss, F. Weinhold, Chem. Rev. 1988, 88, 899–926.