Selective Propene Epoxidation on Immobilized Au6–10 Clusters: The Effect of Hydrogen and Water on Activity and Selectivity†
Work supported by Spanish MEC (MAT2005-06544-C03-01) and JCyL (VA017A08) grants. L.M.M. acknowledges support from the “Ramon y Cajal” program. The work at Argonne National Laboratory was supported by the US Department of Energy, BES-Chemical Sciences, BES-Materials Sciences, and BES-Scientific User Facilities under Contract DE-AC-02-06CH11357 with UChicago Argonne, LLC, Operator of Argonne National Laboratory. S.V. gratefully acknowledges the support by the Air Force Office of Scientific Research. B.H. acknowledges financial support from the Danish research councils, FNU, DSF/NABIIT, and DCSC.
Graphical Abstract
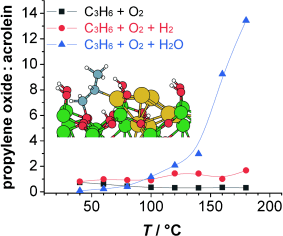
Epoxidation made easy: Subnanometer gold clusters immobilized on amorphous alumina result in a highly active and selective catalyst for propene epoxidation. The highest selectivity is found for gas mixtures involving oxygen and water, thus avoiding the use of hydrogen. Ab initio DFT calculations are used to identify key reaction intermediates and reaction pathways. The results confirm the high catalyst activity owing to the formation of propene oxide metallacycles. Al green, Au yellow, O red, and C gray.
Abstract
Epoxidation made easy: Subnanometer gold clusters immobilized on amorphous alumina result in a highly active and selective catalyst for propene epoxidation. The highest selectivity is found for gas mixtures involving oxygen and water, thus avoiding the use of hydrogen. Ab initio DFT calculations are used to identify key reaction intermediates and reaction pathways. The results confirm the high catalyst activity owing to the formation of propene oxide metallacycles. Al green, Au yellow, O red, and C gray.