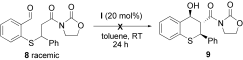A Highly Stereoselective Hydrogen-Bond-Mediated Michael–Michael Cascade Process through Dynamic Kinetic Resolution†
Jian Wang Dr.
Department of Chemistry & Chemical Biology, University of New Mexico, MSC03 2060, Albuquerque, NM 87131-0001, USA, Fax: (+1) 505-277-2609
Search for more papers by this authorHexin Xie
Department of Chemistry & Chemical Biology, University of New Mexico, MSC03 2060, Albuquerque, NM 87131-0001, USA, Fax: (+1) 505-277-2609
Search for more papers by this authorHao Li Dr.
Department of Chemistry & Chemical Biology, University of New Mexico, MSC03 2060, Albuquerque, NM 87131-0001, USA, Fax: (+1) 505-277-2609
Search for more papers by this authorLiansuo Zu
Department of Chemistry & Chemical Biology, University of New Mexico, MSC03 2060, Albuquerque, NM 87131-0001, USA, Fax: (+1) 505-277-2609
Search for more papers by this authorWei Wang Prof. Dr.
Department of Chemistry & Chemical Biology, University of New Mexico, MSC03 2060, Albuquerque, NM 87131-0001, USA, Fax: (+1) 505-277-2609
Search for more papers by this authorJian Wang Dr.
Department of Chemistry & Chemical Biology, University of New Mexico, MSC03 2060, Albuquerque, NM 87131-0001, USA, Fax: (+1) 505-277-2609
Search for more papers by this authorHexin Xie
Department of Chemistry & Chemical Biology, University of New Mexico, MSC03 2060, Albuquerque, NM 87131-0001, USA, Fax: (+1) 505-277-2609
Search for more papers by this authorHao Li Dr.
Department of Chemistry & Chemical Biology, University of New Mexico, MSC03 2060, Albuquerque, NM 87131-0001, USA, Fax: (+1) 505-277-2609
Search for more papers by this authorLiansuo Zu
Department of Chemistry & Chemical Biology, University of New Mexico, MSC03 2060, Albuquerque, NM 87131-0001, USA, Fax: (+1) 505-277-2609
Search for more papers by this authorWei Wang Prof. Dr.
Department of Chemistry & Chemical Biology, University of New Mexico, MSC03 2060, Albuquerque, NM 87131-0001, USA, Fax: (+1) 505-277-2609
Search for more papers by this authorFinancial support of this research was provided by the NSF (CHE-0704015) and the ACS Petroleum Research Fund.
Graphical Abstract
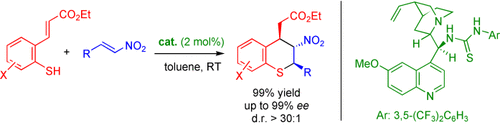
Oh Mickey, you're so fine: The title reaction, which is efficiently catalyzed by a cinchona alkaloid thioure affords direct access to thiochromanes in high efficiency. The reaction features a new activation mode of organocatalytic dynamic kinetic resolution involving a Michael–retro-Michael–Michael–Michael cascade.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2008/z800381_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews of organocatalytic cascade reactions, see:
- 1aD. Enders, C. Grondal, M. R. M. Hüttl, Angew. Chem. 2007, 119, 1590;
10.1002/ange.200603129 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 1570;
- 1bA. M. Walji, D. W. C. MacMillan, Synlett 2007, 1477.
- 2For recent selected examples of cascade reactions catalyzed by chiral amines, see:
- 2aD. B. Ramachary, N. S. Chowdari, C. F. Barbas III, Angew. Chem. 2003, 115, 4365;
10.1002/ange.200351916 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 4233;
- 2bD. B. Ramachary, K. Anebouselvy, N. S. Chowdari, C. F. Barbas III, J. Org. Chem. 2004, 69, 5838;
- 2cY. Yamamoto, N. Momiyama, H. Yamamoto, J. Am. Chem. Soc. 2004, 126, 5962;
- 2dY. Huang, A. M. Walji, C. H. Larsen, D. W. C. MacMillan, J. Am. Chem. Soc. 2005, 127, 15051;
- 2eJ. W. Yang, M. T. H. Fonseca, B. List, J. Am. Chem. Soc. 2005, 127, 15036;
- 2fM. Marigo, T. Schulte, J. Franzen, K. A. Jørgensen, J. Am. Chem. Soc. 2005, 127, 15710;
- 2gD. Enders, M. R. M. Hüttl, C. Grondal, G. Raabe, Nature 2006, 441, 861;
- 2hS. Brandau, E. Maerten, K. A. Jørgensen, J. Am. Chem. Soc. 2006, 128, 14986;
- 2iM. Marigo, S. Bertelsen, A. Landa, K. A. Jørgensen, J. Am. Chem. Soc. 2006, 128, 5475;
- 2jW. Wang, H. Li, J. Wang, L. Zu, J. Am. Chem. Soc. 2006, 128, 10354;
- 2kR. Rios, H. Sunden, I. Ibrahem, G.-L. Zhao, L. Eriksson, A. Córdova, Tetrahedron Lett. 2006, 47, 8547;
- 2lD. Enders, M. R. M. Hüttl, J. Runsink, G. Raabe, B. Wendt, Angew. Chem. 2007, 119, 471;
10.1002/ange.200603434 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 467;
- 2mA. Carlone, S. Cabrera, M. Marigo, K. A. Jørgensen, Angew. Chem. 2007, 119, 1119;
10.1002/ange.200604479 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 1101;
- 2nH. Li, J. Wang, H. Xie, L. Zu, W. Jiang, E. N. Duesler, W. Wang, Org. Lett. 2007, 9, 965;
- 2oH. Sunden, I. Ibrahem, G.-L. Zhao, L. Eriksson, A. Córdova, Chem. Eur. J. 2007, 13, 574;
- 2pH. Li, L. Zu, H. Xie, J. Wang, W. Jiang, W. Wang, Angew. Chem. 2007, 119, 3806; Angew. Chem. Int. Ed. 2007, 46, 3732;
- 2qY. Hayashi, T. Okano, S. Aratake, D. Hazelard, Angew. Chem. 2007, 119, 5010; Angew. Chem. Int. Ed. 2007, 46, 4922;
- 2rJ. L. Vicario, S. Reboredo, D. Badía, L. Carrillo, Angew. Chem. 2007, 119, 5260;
10.1002/ange.200700988 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 5168;
- 2sD. Enders, A. A. Narine, T. Benninghaus, G. Raabe, Synlett 2007, 1667;
- 2tX. Sun, S. Sengupta, J. L. Petersen, H. Wang, J. P. Lewis, X.-D. Shi, Org. Lett. 2007, 9, 4495.
- 3For reviews of hydrogen-bond-mediated catalysis, see:
- 3aS.-K. Tian, Y.-G. Chen, J.-F. Hang, L. Tang, P. McDaid, L. Deng, Acc. Chem. Res. 2004, 37, 621;
- 3bY. Takemoto, Org. Biomol. Chem. 2005, 3, 4299;
- 3cT. Akiyama, J. Itoh, K. Fuchibe, Adv. Synth. Catal. 2006, 348, 999;
- 3dA. G. Doyle, E. N. Jacobsen, Chem. Rev. 2007, 107, 5713;
- 3eT. Akiyama, J. Itoh, K. Fuchibe, Chem. Rev. 2007, 107, 5744;
- 3fX.-H. Yu, W. Wang, Chem. Asian J. 2008, 3, 516.
- 4For examples of hydrogen-bond-catalyzed cascade reactions, see:
- 4aS. Kaneko, T. Yoshino, T. Katoh, S. Terashima, Tetrahedron 1998, 54, 5471;
- 4bY. Hoashi, T. Yabuta, Y. Takemoto, Tetrahedron Lett. 2004, 45, 9185;
- 4cY. Hoashi, T. Yabuta, P. Yuan, H. Miyabe, Y. Takemoto, Tetrahedron 2006, 62, 365;
- 4dY. Wang, X.-F. Liu, L. Deng, J. Am. Chem. Soc. 2006, 128, 3928;
M. Rueping, A. P. Antonchick, T. Theissmann, Angew. Chem. 2006, 118, 3765;
10.1002/ange.200600191 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 3683;
- 4eT. Akiyama, H. Morita, K. Fuchibe, J. Am. Chem. Soc. 2006, 128, 13070;
- 4fJ. Itoh, K. Fuchibe, T. Akiyama, Angew. Chem. 2006, 118, 4914; Angew. Chem. Int. Ed. 2006, 45, 4796;
- 4gB. Wang, F. Wu, Y. Wang, X. Liu, L. Deng, J. Am. Chem. Soc. 2007, 129, 768;
- 4hM. M. Biddle, M. Lin, K. A. Scheidt, J. Am. Chem. Soc. 2007, 129, 3830;
- 4iA. Chan, K. A. Scheidt, J. Am. Chem. Soc. 2007, 129, 5334;
- 4jJ. Zhou, B. List, J. Am. Chem. Soc. 2007, 129, 7498;
- 4kM. Terada, K. Machioka, K. Sorimachi, J. Am. Chem. Soc. 2007, 129, 10336.
- 5
- 5aL.-S. Zu, J. Wang, H. Li, H.-X. Xie, W. Jiang, W. Wang, J. Am. Chem. Soc. 2007, 129, 1036;
- 5bL.-S. Zu, H.-X. Xie, H. Li, J. Wang, W. Jiang, W. Wang, Adv. Synth. Catal. 2007, 349, 1882.
- 6See Table S1 of the Supporting Information for details.
- 7CCDC 675590 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 8The reaction of thiophenol with trans-β-nitrostyrene under the same reaction conditions and in the presence of catalyst I gave only 12 % ee. We found that the process proceeded very quickly even without I.
- 9For recent reviews of dynamic kinetic resolutions, see
- 9aG. R. Cook, Curr. Org. Chem. 2000, 4, 869;
- 9bF. F. Huerta, A. B. E. Minidis, J.-E. Backvall, Chem. Soc. Rev. 2001, 30, 321;
- 9cH. Pellissier, Tetrahedron 2003, 59, 8291.
- 10For examples of organocatalytic dynamic kinetic resolutions, see:
- 10aJ. Liang, J. C. Ruble, G. C. Fu, J. Org. Chem. 1998, 63, 3154;
- 10bJ. Hang, H. Li, L. Deng, Org. Lett. 2002, 4, 3321;
- 10cL. Tang, L. Deng, J. Am. Chem. Soc. 2002, 124, 2870;
- 10dA. Berkessel, F. Cleemann, S. Mukherjee, T. N. Müller, J. Lex, Angew. Chem. 2005, 117, 817;
10.1002/ange.200461442 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 807;
- 10eA. Berkessel, F. Cleemann, S. Mukherjee, Angew. Chem. 2005, 117, 7632;
10.1002/ange.200502003 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 7466;
- 10fS. Hoffmann, M. Nicoletti, B. List, J. Am. Chem. Soc. 2006, 128, 13074;
- 10gY.-J. Wang, Z.-X. Shen, B. Li, Y. Zhang, Y.-W. Zhang, Chem. Commun. 2007, 1284;
- 10hB. J. Cowen, S. J. Miller, J. Am. Chem. Soc. 2007, 129, 10988.
- 11For recent examples of organocatalytic Rauhut–Currier reactions, see:
- 11aL.-C. Wang, A. L. Luis, K. Agapiou, H.-Y. Jang, M. J. Krische, J. Am. Chem. Soc. 2002, 124, 2402;
- 11bS. A. Frank, D. J. Mergott, W. R. Roush, J. Am. Chem. Soc. 2002, 124, 2404;
- 11cD. J. Mergott, S. A. Frank, W. R. Roush, Org. Lett. 2002, 4, 3157;
- 11dK. Agapiou, M. J. Krische, Org. Lett. 2003, 5, 1737;
- 11eC. E. Aroyan, S. J. Miller, J. Am. Chem. Soc. 2007, 129, 256.
- 12Treatment of racemic 8 in the presence of I (20 mol %) in toluene at room temperature did not result in the formation of 9. Instead, only starting material was recovered.