A Concise Approach to Vinigrol†
Thomas J. Maimone
Department of Chemistry, The Scripps Research Institute, 10650 North Torrey Pines Road, La Jolla, CA 92037, USA, Fax: (+1) 858-784-7375
Search for more papers by this authorAna-Florina Voica
Department of Chemistry, The Scripps Research Institute, 10650 North Torrey Pines Road, La Jolla, CA 92037, USA, Fax: (+1) 858-784-7375
Search for more papers by this authorPhil S. Baran Prof.
Department of Chemistry, The Scripps Research Institute, 10650 North Torrey Pines Road, La Jolla, CA 92037, USA, Fax: (+1) 858-784-7375
Search for more papers by this authorThomas J. Maimone
Department of Chemistry, The Scripps Research Institute, 10650 North Torrey Pines Road, La Jolla, CA 92037, USA, Fax: (+1) 858-784-7375
Search for more papers by this authorAna-Florina Voica
Department of Chemistry, The Scripps Research Institute, 10650 North Torrey Pines Road, La Jolla, CA 92037, USA, Fax: (+1) 858-784-7375
Search for more papers by this authorPhil S. Baran Prof.
Department of Chemistry, The Scripps Research Institute, 10650 North Torrey Pines Road, La Jolla, CA 92037, USA, Fax: (+1) 858-784-7375
Search for more papers by this authorWe thank Dr. D.-H. Huang and Dr. L. Pasternack for NMR spectroscopic assistance, Dr. G. Siuzdak for mass spectrometric and Dr. Arnold Rheingold (UCSD) for X-ray crystallographic assistance. Financial support for this work was provided by Bristol-Myers Squibb (predoctoral fellowship to T. J. M.), Merck, and Roche.
Graphical Abstract
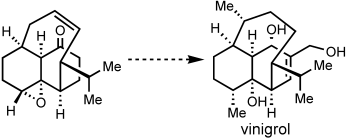
Short and sweet: A simple and practical route to the unusual tricyclic ring system found in the biologically active diterpene vinigrol is described. A remarkable proximity-induced intramolecular cycloaddition and mild Grob fragmentation allow rapid construction of the core ring system in less than 10 steps and with a minimal reliance on protecting groups.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2008/z800167_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1I. Uchida, T. Ando, N. Fukami, K. Yoshida, M. Hashimoto, T. Tada, S. Koda, Y. Morimoto, J. Org. Chem. 1987, 52, 5292–5293.
- 2
- 2aT. Ando, Y. Tsurumi, N. Ohata, I. Uchida, K. Yoshida, M. Okuhara, J. Antibiot. 1988, 41, 25–30;
- 2bT. Ando, K. Yoshida, M. Okuhara, J. Antibiot. 1988, 41, 31–35;
- 2cD. B. Norris, P. Depledge, A. P. Jackson, PCT Int. Appl. WO 9107 953, 1991;
- 2dH. Nakajima, N. Yamamoto, T. Kaizu, T. Kino (Jpn. Kokai Tokkyo Koho), JP 07-206668, 1995.
- 3Studies toward vinigrol:
- 3aL. A. Paquette, R. Guevel, S. Sakamoto, I. H. Kim, J. Crawford, J. Org. Chem. 2003, 68, 6096–6107;
- 3bL. A. Paquette, I. Efremov, Z. S. Liu, J. Org. Chem. 2005, 70, 505–509;
- 3cL. A. Paquette, I. Efremov, J. Org. Chem. 2005, 70, 510–513;
- 3dL. A. Paquette, Z. S. Liu, I. Efremov, J. Org. Chem. 2005, 70, 514–518;
- 3eThis author has also recognized this bond disconnection: S. N. Goodman, Ph.D. Thesis, Harvard University, 2000;
- 3fC. M. Grise, G. Tessier, L. Barriault, Org. Lett. 2007, 9, 1545–1548;
- 3gL. Morency, L. Barriault, J. Org. Chem. 2005, 70, 8841–8853;
- 3hL. Morency, L. Barriault, Tetrahedron Lett. 2004, 45, 6105–6107;
- 3iJ. G. Devaux, I. Hanna, J. Y. Lallemand, J. Org. Chem. 1997, 62, 5062–5068;
- 3jJ. F. Devaux, I. Hanna, J. Y. Lallemand, J. Org. Chem. 1993, 58, 2349–2350;
- 3kL. Gentric, I. Hanna, L. Ricard, Org. Lett. 2003, 5, 1139–1142;
- 3lG. Mehta, K. S. Reddy, Synlett 1996, 625–627;
- 3mM. Kito, T. Sakai, N. Haruta, H. Shirahama, F. Matsuda, Synlett 1996, 1057–1060;
- 3nM. Kito, T. Sakai, H. Shirahama, M. Miyashita, F. Matsuda, Synlett 1997, 219–220;
- 3oF. Matsuda, M. Kito, T. Sakai, N. Okada, M. Miyashita, H. Shirahama, Tetrahedron 1999, 55, 14369–14380;
- 3pM. S. Souweha, G. D. Enright, A. G. Fallis, Org. Lett. 2007, 9, 5163–5166;
- 3qG. Tessier, L. Barriault, Org. Prep. Proc. Int. 2007, 39, 311–353;
- 3rJ.-F. Devaux, I. Hanna, J.-Y. Lallemand, T. Prange, J. Chem. Res. Syn. 1996, 32–33.
- 4T. J. Maimone, P. S. Baran, Nat. Chem. Biol. 2007, 3, 396–407.
- 5H. Lamy-Schelkens, D. Giomi, L. Ghosez, Tetrahedron Lett. 1989, 30, 5887–5890.
- 6K. C. Nicolaou, P. G. Bulger, D. Sarlah, Angew. Chem. 2005, 117, 4516–4563;
10.1002/ange.200500368 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 4442–4489.
- 7For an early example of an intramolecular Diels–Alder reaction between unactivated π systems (160–190 °C), see:
- 7aS. R. Wilson, D. T. Mao, J. Am. Chem. Soc. 1978, 100, 6289–6291. For a review of the Diels–Alder reaction in total synthesis, see:
- 7bK. C. Nicolaou, S. A. Snyder, T. Montagnon, G. Vassilikogiannakis, Angew. Chem. 2002, 114, 1742–1773;
10.1002/1521-3757(20020517)114:10<1742::AID-ANGE1742>3.0.CO;2-Y Google ScholarAngew. Chem. Int. Ed. 2002, 41, 1668–1698. For a general review of intramolecular Diels–Alder reactions, see:10.1002/1521-3773(20020517)41:10<1668::AID-ANIE1668>3.0.CO;2-Z CAS PubMed Web of Science® Google Scholar
- 7cW. R. Roush in Comprehensive Organic Synthesis, Vol. 5 (Ed.: ), Pergamon, Oxford, 1991, pp. 513–550;
10.1016/B978-0-08-052349-1.00131-1 Google Scholar
- 7dG. Brieger, J. N. Bennett, Chem. Rev. 1980, 80, 63–97;
- 7eB. R. Bear, S. M. Sparks, K. J. Shea, Angew. Chem. 2001, 113, 864–894;
10.1002/1521-3757(20010302)113:5<864::AID-ANGE864>3.0.CO;2-0 Google ScholarAngew. Chem. Int. Ed. 2001, 40, 820–849;10.1002/1521-3773(20010302)40:5<820::AID-ANIE820>3.0.CO;2-F CAS PubMed Web of Science® Google Scholar
- 7fK. Takao, R. Munakata, K. Tadano, Chem. Rev. 2005, 105, 4779–4807.
- 8T.-L. Ho, Heterolytic Fragmentation of Organic Molecules, Wiley, New York, 1993.
- 9P. S. Baran, T. J. Maimone, J. M. Richter, Nature 2007, 446, 404–408; As the silyl groups are necessary to maintain the diene form of 8, they are not considered to be a protecting group, for a discussion see: R. W. Hoffmann, Synthesis 2006, 3531–3541.
- 10Full characterization and experimental details can be found in Supporting Information. CCDC-675358, 675356, and 675357 (5, 13, and 15, respectively) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.