Highlight
A Base-Stabilized Neutral BB Bond: Closing a Gap by Filling the Void
David Scheschkewitz Dr.,
David Scheschkewitz Dr.
Julius-Maximilians Universität Würzburg, Institut für Anorganische Chemie, Am Hubland, 97074 Würzburg, Germany, Fax: (+49) 931-888-4623
Search for more papers by this authorDavid Scheschkewitz Dr.,
David Scheschkewitz Dr.
Julius-Maximilians Universität Würzburg, Institut für Anorganische Chemie, Am Hubland, 97074 Würzburg, Germany, Fax: (+49) 931-888-4623
Search for more papers by this authorGraphical Abstract
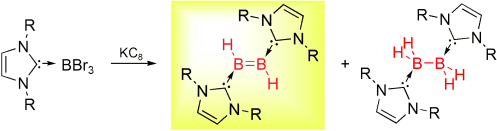
The lightest double bond, that is, the one between two atoms of boron, was finally realized in an electroneutral compound. Robinson et al. satisfied the electron demand of HBBH through coordination of two molecules of an N-heterocyclic carbene (see scheme). As by-product, the base-stabilized parent diborane(4), the formal hydrogenation product of the title compound, was isolated.
References
- 1Y. Wang, B. Quillian, P. Wei, C. S. Wannere, Y. Xie, R. B. King, H. F. Schaefer III, P. von R. Schleyer, G. H. Robinson, J. Am. Chem. Soc. 2007, 129, 12412.
- 2Selected reviews:
- 2aE. Rivard, P. P. Power, Inorg. Chem. 2007, 46, 10047;
- 2bM. Kira, T. Iwamoto, Adv. Organomet. Chem. 2006, 54, 73;
- 2cA. H. Cowley, J. Organomet. Chem. 2004, 689, 3866;
- 2dM. Weidenbruch, Organometallics 2003, 22, 4348;
- 2eV. Y. Lee, A. Sekiguchi, Organometallics 2004, 23, 2822;
- 2fC. Jones, Coord. Chem. Rev. 2001, 215, 151;
- 2gN. Tokitoh, Pure Appl. Chem. 1999, 71, 495;
- 2hM. Yoshifuji, Pure Appl. Chem. 1999, 71, 503;
- 2iP. P. Power, Chem. Rev. 1999, 99, 3463.
- 3Recent reviews:
- 3aZ. Chen, R. B. King, Chem. Rev. 2005, 105, 3613;
- 3bN. S. Hosmane, Pure Appl. Chem. 2003, 75, 1219;
- 3cS. L. Shea, J. Bould, M. G. S. Londesborough, S. D. Perera, A. Franken, D. L. Ormsby, T. Jelínek, B. Štíbr, J. Holub, C. A. Kilner, M. Thornton-Pett, J. D. Kennedy, Pure Appl. Chem. 2003, 75, 1239.
- 4Reviews:
- 4aJ. J. Eisch, Adv. Organomet. Chem. 1996, 39, 355;
- 4bA. Berndt, Angew. Chem. 1993, 105, 1034; Angew. Chem. Int. Ed. Engl. 1993, 32, 985.
- 5Reviews:
- 5aP. Paetzold, Adv. Inorg. Chem. 1987, 31, 123;
- 5bH. Nöth, Angew. Chem. 1988, 100, 1664; Angew. Chem. Int. Ed. Engl. 1988, 27, 1603.
- 6Reviews:
- 6aH. Braunschweig, C. Kollann, D. Rais, Angew. Chem. 2006, 118, 5380; Angew. Chem. Int. Ed. 2006, 45, 5254;
- 6bH. Braunschweig, D. Rais, Heteroat. Chem. 2005, 16, 566;
- 6cS. Aldridge, D. L. Coombs, Coord. Chem. Rev. 2004, 248, 535.
- 7J. Allwohn, M. Pilz, R. Hunold, W. Massa, A. Berndt, Angew. Chem. 1990, 102, 1084; Angew. Chem. Int. Ed. Engl. 1990, 29, 1032.
- 8
- 8aH. Klusik, A. Berndt, Angew. Chem. 1981, 93, 903; Angew. Chem. Int. Ed. Engl. 1981, 20, 870;
- 8bW. J. Grigsby, P. P. Power, Chem. Eur. J. 1997, 3, 368.
- 9E. Kaufmann, P. von R. Schleyer, Inorg. Chem. 1988, 27, 3987.
- 10aA. Moezzi, M. M. Olmstead, P. P. Power, J. Am. Chem. Soc. 1992, 114, 2715;
- 10bA. Moezzi, R. A. Bartlett, P. P. Power, Angew. Chem. 1992, 104, 1075; Angew. Chem. Int. Ed. Engl. 1992, 31, 1082;
- 10cP. P. Power, Inorg. Chim. Acta 1992, 198–200, 443;
- 10dH. Nöth, J. Knizek, W. Ponikwar, Eur. J. Inorg. Chem. 1999, 1931.
10.1002/(SICI)1099-0682(199911)1999:11<1931::AID-EJIC1931>3.0.CO;2-D CAS Web of Science® Google Scholar
- 11Two electron aromatics:
- 11aR. Wehrmann, H. Meyer, A. Berndt, Angew. Chem. 1985, 97, 779; Angew. Chem. Int. Ed. Engl. 1985, 24, 788;
- 11bC. Präsang, A. Mlodzianowska, G. Geiseler, W. Massa, M. Hofmann, A. Berndt, Pure Appl. Chem. 2003, 75, 1175;
- 11cM. Hofmann, A. Berndt, Heteroat. Chem. 2006, 17, 224.
- 12Six-electron aromatics:
- 12aG. E. Herberich, B. Heßner, M. Hostalek, Angew. Chem. 1986, 98, 637; Angew. Chem. Int. Ed. Engl. 1986, 25, 642;
- 12bD. Scheschkewitz, M. Menzel, M. Hofmann, P. von R. Schleyer, G. Geiseler, W. Massa, K. Harms, A. Berndt, Angew. Chem. 1999, 111, 3116;
10.1002/(SICI)1521-3757(19991004)111:19<3116::AID-ANGE3116>3.0.CO;2-G Google ScholarAngew. Chem. Int. Ed. 1999, 38, 2936.10.1002/(SICI)1521-3773(19991004)38:19<2936::AID-ANIE2936>3.0.CO;2-9 CAS PubMed Web of Science® Google Scholar
- 13C. Präsang, A. Mlodzianowska, Y. Sahin, G. Geiseler, W. Massa, M. Hofmann, A. Berndt, Angew. Chem. 2002, 114, 3529;
10.1002/1521-3757(20020916)114:18<3529::AID-ANGE3529>3.0.CO;2-O Google ScholarAngew. Chem. Int. Ed. 2002, 41, 3380.10.1002/1521-3773(20020916)41:18<3380::AID-ANIE3380>3.0.CO;2-8 CAS PubMed Web of Science® Google Scholar
- 14R. J. Wright, A. D. Phillips, P. P. Power, J. Am. Chem. Soc. 2003, 125, 10784.
- 15
- 15aT. Mennekes, P. Paetzold, R. Boese, Angew. Chem. 1990, 102, 909; Angew. Chem. Int. Ed. Engl. 1990, 29, 899;
- 15bW. J. Grigsby, P. P. Power, J. Am. Chem. Soc. 1996, 118, 7981.
- 16
- 16aJ. D. Dill, P. von R. Schleyer, J. A. Pople, J. Am. Chem. Soc. 1975, 97, 3402;
- 16bG. Treboux, J.-C. Barthelat, J. Am. Chem. Soc. 1993, 115, 4870;
- 16cE. D. Jemmis, B. Pathak, R. B. King, H. F. Schaefer III, Chem. Commun. 2006, 2164.
- 17L. B. Knight, Jr., K. Kerr, P. K. Miller, C. A. Arrington, J. Phys. Chem. 1995, 99, 16842.
- 18C. Jouany, J. C. Barthelat, J. P. Daudey, Chem. Phys. Lett. 1987, 136, 52.
- 19
- 19aA. Meller, W. Maringgele in Advances in Boron Chemistry (Ed.: ), Royal Society of Chemistry, Cambridge, 1997, pp. 224–231;
- 19bC.-J. Maier, H. Pritzkow, W. Siebert, Angew. Chem. 1999, 111, 1772;
10.1002/(SICI)1521-3757(19990601)111:11<1772::AID-ANGE1772>3.0.CO;2-X Google ScholarAngew. Chem. Int. Ed. 1999, 38, 1666.10.1002/(SICI)1521-3773(19990601)38:11<1666::AID-ANIE1666>3.0.CO;2-V CAS PubMed Web of Science® Google Scholar
- 20
- 20aJ. Su, X.-W. Li, R. C. Crittendon, G. H. Robinson, J. Am. Chem. Soc. 1997, 119, 5471;
- 20bK. W. Klinkhammer, Angew. Chem. 1997, 109, 2414;
10.1002/ange.19971092107 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 2320;
- 20cF. A. Cotton, A. H. Cowley, X. Feng, J. Am. Chem. Soc. 1998, 120, 1795;
- 20dI. Bytheway, Z. Lin, J. Am. Chem. Soc. 1998, 120, 12133;
- 20eT. L. Allen, W. H. Fink, P. P. Power, J. Chem. Soc. Dalton Trans. 2000, 407.
- 21Z.-X. Wang, Z. Chen, H. Jiao, P. von R. Schleyer, J. Theor. Comput. Chem. 2005, 4, 669.