Conformational Properties of 4-Mercaptoproline and Related Derivatives†
Sergio A. Cadamuro Dr.
Max-Planck-Institut für Biochemie, Am Klopferspitz 18, 82152 Martinsried, Germany, Fax: (+49) 89-8578-2847 http://www.biochem.mpg.de/en/rg/moroder/
Search for more papers by this authorRudolf Reichold
Lehrstuhl für Biomolekulare Optik, Ludwig-Maximilians-Universität, Oettingenstrasse 67, 80538 München, Germany
Search for more papers by this authorUlrike Kusebauch Dr.
Max-Planck-Institut für Biochemie, Am Klopferspitz 18, 82152 Martinsried, Germany, Fax: (+49) 89-8578-2847 http://www.biochem.mpg.de/en/rg/moroder/
Search for more papers by this authorHans-Jürgen Musiol
Max-Planck-Institut für Biochemie, Am Klopferspitz 18, 82152 Martinsried, Germany, Fax: (+49) 89-8578-2847 http://www.biochem.mpg.de/en/rg/moroder/
Search for more papers by this authorChristian Renner Priv.-Doz. Dr.
Max-Planck-Institut für Biochemie, Am Klopferspitz 18, 82152 Martinsried, Germany, Fax: (+49) 89-8578-2847 http://www.biochem.mpg.de/en/rg/moroder/
Current address: Deutsche Forschungsgemeinschaft, Kennedyallee 40, 53170 Bonn/Bad-Godesberg, Germany
Search for more papers by this authorPaul Tavan Prof. Dr.
Lehrstuhl für Biomolekulare Optik, Ludwig-Maximilians-Universität, Oettingenstrasse 67, 80538 München, Germany
Search for more papers by this authorLuis Moroder Prof. Dr.
Max-Planck-Institut für Biochemie, Am Klopferspitz 18, 82152 Martinsried, Germany, Fax: (+49) 89-8578-2847 http://www.biochem.mpg.de/en/rg/moroder/
Search for more papers by this authorSergio A. Cadamuro Dr.
Max-Planck-Institut für Biochemie, Am Klopferspitz 18, 82152 Martinsried, Germany, Fax: (+49) 89-8578-2847 http://www.biochem.mpg.de/en/rg/moroder/
Search for more papers by this authorRudolf Reichold
Lehrstuhl für Biomolekulare Optik, Ludwig-Maximilians-Universität, Oettingenstrasse 67, 80538 München, Germany
Search for more papers by this authorUlrike Kusebauch Dr.
Max-Planck-Institut für Biochemie, Am Klopferspitz 18, 82152 Martinsried, Germany, Fax: (+49) 89-8578-2847 http://www.biochem.mpg.de/en/rg/moroder/
Search for more papers by this authorHans-Jürgen Musiol
Max-Planck-Institut für Biochemie, Am Klopferspitz 18, 82152 Martinsried, Germany, Fax: (+49) 89-8578-2847 http://www.biochem.mpg.de/en/rg/moroder/
Search for more papers by this authorChristian Renner Priv.-Doz. Dr.
Max-Planck-Institut für Biochemie, Am Klopferspitz 18, 82152 Martinsried, Germany, Fax: (+49) 89-8578-2847 http://www.biochem.mpg.de/en/rg/moroder/
Current address: Deutsche Forschungsgemeinschaft, Kennedyallee 40, 53170 Bonn/Bad-Godesberg, Germany
Search for more papers by this authorPaul Tavan Prof. Dr.
Lehrstuhl für Biomolekulare Optik, Ludwig-Maximilians-Universität, Oettingenstrasse 67, 80538 München, Germany
Search for more papers by this authorLuis Moroder Prof. Dr.
Max-Planck-Institut für Biochemie, Am Klopferspitz 18, 82152 Martinsried, Germany, Fax: (+49) 89-8578-2847 http://www.biochem.mpg.de/en/rg/moroder/
Search for more papers by this authorThis work was supported by the Deutsche Forschungsgemeinschaft (SFB 533, A8, and C3).
Graphical Abstract
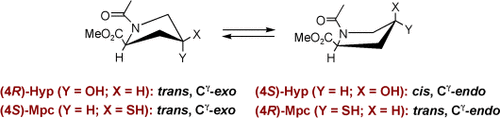
Spot the difference: Conformational analysis of the 2S,4R and 2S,4S epimers of N-acetyl-4-mercaptopyrrolidine-2-carboxylic acid methyl esters reveals ring-pucker preferences that are opposite of those of the hydroxyproline derivatives (see scheme). Replacement of proline or hydroxyproline in polypeptides with the chalcogen analogue should allow for fine-tuning of the complex interplay of noncovalent interactions, steric hindrance, and stereoelectronic effects.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2008/z704310_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. F. Brandts, H. R. Halvorson, M. Brennan, Biochemistry 1975, 14, 4953–4963;
- 1bW. J. Wedemeyer, E. Welker, H. A. Scheraga, Biochemistry 2002, 41, 14637–14644;
- 1cG. Fischer, Chem. Soc. Rev. 2000, 29, 119–127;
- 1dF. X. Schmid in Protein Folding Handbook, Vol. 2 (Eds: ), Wiley-VCH, Weinheim, 2005, pp. 916–945.
- 2
- 2aE. S. Eberhardt, N. Panasik, R. T. Raines, J. Am. Chem. Soc. 1996, 118, 12261–12266;
- 2bN. Panasik, E. S. Eberhardt, A. S. Edison, D. R. Powell, R. T. Raines, Int. J. Pept. Protein Res. 1994, 44, 262–269;
- 2cD. Kern, M. Schutkowski, T. Drakenberg, J. Am. Chem. Soc. 1997, 119, 8403–8408;
- 2dM. Keller, C. Sager, P. Dumy, M. Schutkowski, G. S. Fischer, M. Mutter, J. Am. Chem. Soc. 1998, 120, 2714–2720;
- 2eE. Beausoleil, R. Sharma, S. W. Michnick, W. D. Lubell, J. Org. Chem. 1998, 63, 6572–6578;
- 2fS. S. A. An, C. C. Lester, J. L. Peng, Y. J. Li, D. M. Rothwarf, E. Welker, T. W. Thannhauser, L. S. Zhang, J. P. Tam, H. A. Scheraga, J. Am. Chem. Soc. 1999, 121, 11558–11566;
- 2gC. Renner, S. Alefelder, J. H. Bae, N. Budisa, R. Huber, L. Moroder, Angew. Chem. 2001, 113, 949–951;
10.1002/1521-3757(20010302)113:5<949::AID-ANGE949>3.0.CO;2-L Google ScholarAngew. Chem. Int. Ed. 2001, 40, 923–925;10.1002/1521-3773(20010302)40:5<923::AID-ANIE923>3.0.CO;2-# CAS PubMed Web of Science® Google Scholar
- 2hM. L. DeRider, S. J. Wilkens, M. J. Waddell, L. E. Bretscher, F. Weinhold, R. T. Raines, J. L. Markley, J. Am. Chem. Soc. 2002, 124, 2497–2505;
- 2iJ. A. Hodges, R. T. Raines, J. Am. Chem. Soc. 2003, 125, 9262–9263;
- 2jY. Che, G. R. Marshall, J. Org. Chem. 2004, 69, 9030–9042;
- 2kL. E. Bretscher, C. L. Jenkins, K. M. Taylor, M. L. DeRider, R. T. Raines, J. Am. Chem. Soc. 2001, 123, 777–778;
- 2lC. M. Taylor, R. Hardre, P. J. B. Edwards, J. Org. Chem. 2005, 70, 1306–1315;
- 2mW. Kim, K. I. Hardcastle, V. P. Conticello, Angew. Chem. 2006, 118, 8321–8325; Angew. Chem. Int. Ed. 2006, 45, 8141–8145;
- 2nM. D. Shoulders, J. A. Hodges, R. T. Raines, J. Am. Chem. Soc. 2006, 128, 8112–8113;
- 2oS. K. M. Umashankara, I. R. Babu, K. N. Ganesh, Chem. Commun. 2003, 2606–2607;
- 2pI. R. Babu, K. N. Ganesh, J. Am. Chem. Soc. 2001, 123, 2079–2080.
- 3
- 3aI. Wagner, H. Musso, Angew. Chem. 1983, 95, 827–839; Angew. Chem. Int. Ed. Engl. 1983, 22, 816–828;
- 3bA. B. Mauger, J. Nat. Prod. 1996, 59, 1205–1211;
- 3cL. Moroder, C. Renner, J. J. Lopez, M. T. Mutter, G. Tuchscherer in Cis-trans Isomerization in Biochemistry (Ed.: ), Wiley-VCH, Weinheim, 2006, pp. 225–259.
10.1002/9783527609338.ch11 Google Scholar
- 4
- 4aS. K. Holmgren, K. M. Taylor, L. E. Bretscher, R. T. Raines, Nature 1998, 392, 666–667;
- 4bS. K. Holmgren, L. E. Bretscher, K. M. Taylor, R. T. Raines, Chem. Biol. 1999, 6, 63–70;
- 4cC. L. Jenkins, L. E. Bretscher, I. A. Guzei, R. T. Raines, J. Am. Chem. Soc. 2003, 125, 6422–6427;
- 4dJ. A. Hodges, R. T. Raines, J. Am. Chem. Soc. 2005, 127, 15923–15932;
- 4eM. Doi, Y. Nishi, S. Uchiyama, Y. Nishiuchi, H. Nishio, T. Nakazawa, T. Ohkubo, Y. Kobayashi, J. Pept. Sci. 2005, 11, 609–616;
- 4fM. Doi, Y. Nishi, S. Uchiyama, Y. Nishiuchi, T. Nakazawa, T. Ohkubo, Y. Kobayashi, J. Am. Chem. Soc. 2003, 125, 9922–9923;
- 4gK. Kawahara, Y. Nishi, S. Nakamura, S. Uchiyama, Y. Nishiuchi, T. Nakazawa, T. Ohkubo, Y. Kobayashi, Biochemistry 2005, 44, 15812–15822;
- 4hY. Nishi, S. Uchiyama, M. Doi, Y. Nishiuchi, T. Nakazawa, Z. Ohkubo, Y. Kobayashi, Biochemistry 2005, 44, 6034–6042;
- 4iD. Barth, A. G. Milbradt, C. Renner, L. Moroder, ChemBioChem 2004, 5, 79–86;
- 4jM. Schumacher, K. Mizuno, H. P. Bächinger, J. Biol. Chem. 2005, 280, 20397–20403;
- 4kA. V. Persikov, J. A. M. Ramshaw, A. Kirkpatrick, B. Brodsky, J. Am. Chem. Soc. 2003, 125, 11500–11501.
- 5
- 5aW. Kim, R. A. McMillan, J. P. Snyder, V. P. Conticello, J. Am. Chem. Soc. 2005, 127, 18121–18132;
- 5bR. Golbik, C. Yu, E. Weyher-Stingl, R. Huber, L. Moroder, N. Budisa, C. Schiene-Fischer, Biochemistry 2005, 44, 16026–16034;
- 5cS. C. R. Lummis, D. L. Beene, L. W. Lee, H. A. Lester, R. W. Broadhurst, D. A. Dougherty, Nature 2005, 438, 248–252.
- 6
- 6aV. Eswarakrishnan, L. Field, J. Org. Chem. 1981, 46, 4182–4187;
- 6bA. J. Verbiscar, B. Witkop, J. Org. Chem. 1970, 35, 1924–1927.
- 7
- 7aD. S. Kemp, J. H. Rothman, T. C. Curran, D. E. Blanchard, Tetrahedron Lett. 1995, 36, 3809–3812;
- 7bG. V. Nikiforovich, J. L. F. Kao, K. Plucinska, W. J. Zhang, G. R. Marshall, Biochemistry 1994, 33, 3591–3598;
- 7cK. Plucinska, T. Kataoka, M. Yodo, W. L. Cody, J. X. He, C. Humblet, G. H. Lu, E. Lunney, T. C. Major, R. L. Panek, P. Schelkun, R. Skeean, G. R. Marshall, J. Med. Chem. 1993, 36, 1902–1913;
- 7dT. Kataoka, D. D. Beusen, J. D. Clark, M. Yodo, G. R. Marshall, Biopolymers 1992, 32, 1519–1533.
- 8C. Grathwohl, K. Wüthrich, Biopolymers 1976, 15, 2025–2041.
- 9G. B. Liang, C. J. Rito, S. H. Gellman, J. Am. Chem. Soc. 1992, 114, 4440–4442.
- 10J. L. Flippen-Anderson, R. Gilardi, I. L. Karle, M. H. Frey, S. J. Opella, L. M. Gierasch, M. Goodman, V. Madison, N. G. Delaney, J. Am. Chem. Soc. 1983, 105, 6609–6614.
- 11U. Kusebauch, S. A. Cadamuro, H. J. Musiol, M. O. Lenz, J. Wachtveitl, L. Moroder, C. Renner, Angew. Chem. 2006, 118, 7170–7173;
10.1002/ange.200601432 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 7015–7018.
- 12
- 12aV. Nagarajan, S. Kamitori, K. Okuyama, J. Biochem. 1999, 125, 310–318;
- 12bR. Z. Kramer, L. Vitagliano, J. Bella, R. Berisio, L. Mazzarella, B. Brodsky, A. Zagari, H. M. Berman, J. Mol. Biol. 1998, 280, 623–638;
- 12cR. Berisio, L. Vitagliano, L. Mazzarella, A. Zagari, Protein Sci. 2002, 11, 262–270;
- 12dL. Vitagliano, R. Berisio, A. Mastrangelo, L. Mazzarella, A. Zagari, Protein Sci. 2001, 10, 2627–2632;
- 12eL. Vitagliano, R. Berisio, L. Mazzarella, A. Zagari, Biopolymers 2001, 58, 459–464.
10.1002/1097-0282(20010415)58:5<459::AID-BIP1021>3.0.CO;2-V CAS PubMed Web of Science® Google Scholar
- 13R. Improta, C. Benzi, V. Barone, J. Am. Chem. Soc. 2001, 123, 12568–12577.
- 14R. T. Raines, Protein Sci. 2006, 15, 1219–1225.
- 15K. Wütrich, NMR of Proteins and Nucleic Acids, Wiley, New York, 1986.
- 16J. T. Gerig, R. S. McLeod, J. Am. Chem. Soc. 1973, 95, 5725–5729.
- 17A. Navarro-Vazquez, J. C. Cobas, F. J. Sardina, J. Casanueva, E. Diez, J. Chem. Inf. Comput. Sci. 2004, 44, 1680–1685.
- 18
- 18aR. Ahlrichs, M. Bär, M. Häser, H. Horn, C. Kölmel, Chem. Phys. Lett. 1989, 162, 165–169;
- 18bO. Treutler, R. Ahlrichs, J. Chem. Phys. 1995, 102, 346–354.
- 19
- 19aA. D. Becke, J. Chem. Phys. 1993, 98, 5648–5652;
- 19bC. T. Lee, W. T. Yang, R. G. Parr, Phys. Rev. B 1988, 37, 785–789.
- 20A. Schäfer, C. Huber, R. Ahlrichs, J. Chem. Phys. 1994, 100, 5829–5835.
- 21A. Klamt, G. Schüürmann, J. Chem. Soc. Perkin Trans. 2 1993, 799–805.
- 22R. S. Mulliken, J. Chem. Phys. 1955, 23, 1833–1840.