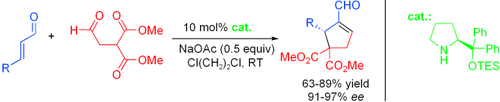Organocatalytic Enantioselective Cascade Michael–Aldol Condensation Reactions: Efficient Assembly of Densely Functionalized Chiral Cyclopentenes†
Jian Wang Dr.
Department of Chemistry & Chemical Biology, University of New Mexico, MSC03 2060, Albuquerque, NM 87131-0001, USA, Fax: (+1) 505-277-2609
Search for more papers by this authorHao Li
Department of Chemistry & Chemical Biology, University of New Mexico, MSC03 2060, Albuquerque, NM 87131-0001, USA, Fax: (+1) 505-277-2609
Search for more papers by this authorHexin Xie
Department of Chemistry & Chemical Biology, University of New Mexico, MSC03 2060, Albuquerque, NM 87131-0001, USA, Fax: (+1) 505-277-2609
Search for more papers by this authorLiansuo Zu
Department of Chemistry & Chemical Biology, University of New Mexico, MSC03 2060, Albuquerque, NM 87131-0001, USA, Fax: (+1) 505-277-2609
Search for more papers by this authorXu Shen Prof. Dr.
Department of Pharmacology, State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China
School of Pharmacy, East China University of Science & Technology, Shanghai 200237, China
Search for more papers by this authorWei Wang Prof. Dr.
Department of Chemistry & Chemical Biology, University of New Mexico, MSC03 2060, Albuquerque, NM 87131-0001, USA, Fax: (+1) 505-277-2609
Department of Pharmacology, State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China
Search for more papers by this authorJian Wang Dr.
Department of Chemistry & Chemical Biology, University of New Mexico, MSC03 2060, Albuquerque, NM 87131-0001, USA, Fax: (+1) 505-277-2609
Search for more papers by this authorHao Li
Department of Chemistry & Chemical Biology, University of New Mexico, MSC03 2060, Albuquerque, NM 87131-0001, USA, Fax: (+1) 505-277-2609
Search for more papers by this authorHexin Xie
Department of Chemistry & Chemical Biology, University of New Mexico, MSC03 2060, Albuquerque, NM 87131-0001, USA, Fax: (+1) 505-277-2609
Search for more papers by this authorLiansuo Zu
Department of Chemistry & Chemical Biology, University of New Mexico, MSC03 2060, Albuquerque, NM 87131-0001, USA, Fax: (+1) 505-277-2609
Search for more papers by this authorXu Shen Prof. Dr.
Department of Pharmacology, State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China
School of Pharmacy, East China University of Science & Technology, Shanghai 200237, China
Search for more papers by this authorWei Wang Prof. Dr.
Department of Chemistry & Chemical Biology, University of New Mexico, MSC03 2060, Albuquerque, NM 87131-0001, USA, Fax: (+1) 505-277-2609
Department of Pharmacology, State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China
Search for more papers by this authorFinancial support of this research by the University of New Mexico, ACS-PRF, NSF (CHE 0704015), and NIH-INBRE program (P20 RR016480) is gratefully acknowledged. Thanks are expressed to Dr. Elieen N. Duesler for performing X-ray analysis.
Graphical Abstract
A cascade of possibility: A novel and highly enantioselective cascade Michael–aldol condensation reaction of α,β-unsaturated aldehydes with dimethyl malonate aldehyde has been developed. The process, efficiently catalyzed by a simple chiral diphenylprolinol TES ether, serves as a powerful approach to the preparation of highly functionalized chiral cyclopentenes with the formation of two new CC bonds. TES=triethylsilyl.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2007/z703163_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Reviews of synthesis and bioactivities of cyclopentenes:
- 1aF. C. Biaggio, A. R. Rufino, M. H. Zaim, C. Y. H. Zaim, M. A. Bueno, A. Rodrigues, Curr. Org. Chem. 2005, 9, 419;
- 1bG. Helmchen, M. Ernst, G. Paradies, Pure Appl. Chem. 2004, 76, 495;
- 1cL. F. Silva, Tetrahedron 2002, 58, 9137;
- 1dW. Kirmse, Angew. Chem. 1997, 109, 1212;
10.1002/ange.19971091105 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 1164;
- 1eM. Lautens, W. Klute, W. Tam, Chem. Rev. 1996, 96, 49;
- 1fC. E. Masse, J. S. Panek, Chem. Rev. 1995, 95, 1293;
- 1gT. Hudlicky, J. D. Price, Chem. Rev. 1989, 89, 1467;
- 1hB. M. Trost, Angew. Chem. 1986, 98, 1; Angew. Chem. Int. Ed. Engl. 1986, 25, 1.
- 2For selected examples of cyclopentenes synthesis, see:
- 2aM. Wadamoto, E. M. Phillips, T. E. Reynolds, K. A. Scheidt, J. Am. Chem. Soc. 2007, 129, 10098;
- 2bM. Rueping, W. Leawsuwan, A. P. Antonchick, B. J. Nachtsheim, Angew. Chem. 2007, 119, 2143;
10.1002/ange.200604809 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 2097;
- 2cS. T. Staben, J. J. Kennedy-Smith, D. Huang, B. K. Corkey, R. L. LaLonde, F. D. Toste, Angew. Chem. 2006, 118, 6137;
10.1002/ange.200602035 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 5991;
- 2dV. Nair, S. Vellalath, M. Poonoth, E. Suresh, J. Am. Chem. Soc. 2006, 128, 8736;
- 2eM. K. Pallerla, J. M. Fox, Org. Lett. 2005, 7, 3593;
- 2fG. Liang, D. Trauner, J. Am. Chem. Soc. 2004, 126, 9544;
- 2gW. He, X. Sun, A. J. Frontier, J. Am. Chem. Soc. 2003, 125, 14278;
- 2hH. M. L. Davies, N. Kong, M. R. Churchill, J. Org. Chem. 1998, 63, 6586;
- 2iJ. S. Panek, N. F. Jain, J. Org. Chem. 1993, 58, 2345.
- 3For reviews, see:
- 3aJ. M. Fox, N. Yan, Curr. Org. Chem. 2005, 9, 719;
- 3bH.-U. Reissig, R. Zimmer, Chem. Rev. 2003, 103, 1151;
- 3cJ. Pietruszka, Chem. Rev. 2003, 103, 1051;
- 3dW. A. Donaldson, Tetrahedron 2001, 57, 8589.
- 4For reviews, see:
- 4aJ. C. Conrad, D. E. Fogg, Curr. Org. Chem. 2006, 10, 185;
- 4bS. F. Martin, Pure Appl. Chem. 2005, 77, 1207;
- 4cM. E. Maier, Angew. Chem. 2000, 112, 2153;
10.1002/1521-3757(20000616)112:12<2153::AID-ANGE2153>3.0.CO;2-X Google ScholarAngew. Chem. Int. Ed. 2000, 39, 2073;10.1002/1521-3773(20000616)39:12<2073::AID-ANIE2073>3.0.CO;2-0 CAS PubMed Web of Science® Google Scholar
- 4dR. H. Grubbs, S. J. Miller, G. C. Fu, Acc. Chem. Res. 1995, 28, 446.
- 5For reviews, see:
- 5aA. J. Frontier, C. Collison, Tetrahedron 2005, 61, 7577;
- 5bK. L. Habermas, S. E. Demark, T. K. Jones, Org. React. 1994, 45, 1;
- 5cC. Santelli-Rouvier, M. Santelli, Synthesis 1983, 429.
- 6For recent reviews of cascade reactions, see:
- 6aK. C. Nicolaou, D. J. Edmonds, P. G. Bulger, Angew. Chem. 2006, 118, 7292;
10.1002/ange.200601872 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 7134;
- 6bH. Guo, J. Ma, Angew. Chem. 2006, 118, 362; Angew. Chem. Int. Ed. 2006, 45, 354;
- 6cH. Pellissier, Tetrahedron 2006, 62, 2143;
- 6dL. F. Tietze, N. Rackelmann, Pure Appl. Chem. 2004, 76, 1967.
- 7Recent reviews of organocatalysis, see:
- 7aA. Berkessel, H. Groger, Asymmetric Organocatalysis: From Biomimetic Concepts to Applications in Asymmetric Synthesis, Wiley, Weinheim, 2005;
- 7bP. I. Dalko, L. Moisan, Angew. Chem. 2004, 116, 5248;
10.1002/ange.200400650 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 5138.
- 8For a review of organocatalytic cascade reactions, see: D. Enders, C. Grondal, M. R. M. Hüttl, Angew. Chem. 2007, 119, 1590;
10.1002/ange.200603129 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 1570.
- 9Recently, our group has reported catalytic, asymmetric cascade reactions; for selected examples, see:
- 9aW. Wang, H. Li, J. Wang, L.-S. Zu, J. Am. Chem. Soc. 2006, 128, 10354;
- 9bL.-S. Zu, J. Wang, H. Li, H.-X. Xie, W. Jiang, W. Wang, J. Am. Chem. Soc. 2007, 129, 1036;
- 9cL.-S. Zu, H. Li, H.-X. Xie, J. Wang, W. Jiang, Y. Tang, W. Wang, Angew. Chem. 2007, 119, 3806;
10.1002/ange.200700485 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 3732;
- 9dH.-X. Xie, L.-S. Zu, H. Li, J. Wang; W. Wang, J. Am. Chem. Soc. 2007, 129, 10886; W. Wang, J. Am. Chem. Soc. 2007, 129, 10886;
- 9eR. Rios, H. Sunden, I. Ibrahem, G.-L. Zhao, L. Eriksson, A. Córdova, Tetrahedron Lett. 2006, 47, 8547;
- 9fH. Sundén, I. Ibrahem, G.-L. Zhao, L. Eriksson, A. Córdova, Chem. Eur. J. 2007, 13, 574.
- 10For selected examples of organocatalytic cascade reactions, see:
- 10aB. D. Ramachary, N. S. Chowdari, C. F. Barbas III, Angew. Chem. 2003, 115, 4365;
10.1002/ange.200351916 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 4233;
- 10bY. Yamamoto, N. Momiyama, H. Yamamoto, J. Am. Chem. Soc. 2004, 126, 5962;
- 10cY. Huang, M. C. Walji, H. Larsen, D. W. C. MacMillan, J. Am. Chem. Soc. 2005, 127, 15051;
- 10dJ. W. Yang, M. T. H. Fonseca, B. List, J. Am. Chem. Soc. 2005, 127, 15036;
- 10eM. Marigo, T. Schulte, J. Franzen, K. A. Jørgensen, J. Am. Chem. Soc. 2005, 127, 15710;
- 10fD. Enders, M. R. M. Huttl, C. Grondal, G. Raabe, Nature 2006, 441, 861;
- 10gM. Marigo, S. Bertelsen, A. Landa, K. A. Jørgensen, J. Am. Chem. Soc. 2006, 128, 5475;
- 10hC. E. Aroyan, S. J. Miller, J. Am. Chem. Soc. 2007, 129, 256;
- 10iB. Wang, F. Wu, Y. Wang, X. Liu, L. Deng, J. Am. Chem. Soc. 2007, 129, 768;
- 10jY. Hayashi, T. Okano, S. Aratake, D. Hazelard, Angew. Chem. 2007, 119, 5010; Angew. Chem. Int. Ed. 2007, 46, 4922;
- 10kJ. L. Vicario, S. Reboredo, D. Badía, L. Carrillo, Angew. Chem. 2007, 119, 5260;
10.1002/ange.200700988 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 5168.
- 11For a review of reactions catalyzed by chiral pyrrolinol ethers, see: C. Palomo, A. Mielgo, Angew. Chem. 2006, 118, 8042; Angew. Chem. Int. Ed. 2006, 45, 7876.
- 12For leading studies of reactions catalyzed by chiral pyrrolinol ethers, see:
- 12aM. Marigo, T. C. Wabnitz, D. Fielenbach, K. A. Jørgensen, Angew. Chem. 2005, 117, 804;
10.1002/ange.200462101 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 794;
- 12bY. Hayashi, H. Gotoh, T. Hayashi, M. Shoji, Angew. Chem. 2005, 117, 4284; Angew. Chem. Int. Ed. 2005, 44, 4212;
- 12cY. Chi, S. H. Gellman, J. Am. Chem. Soc. 2006, 128, 6804.
- 13The structure of the compound derived from molecule 3 f was determined by X-ray crystal analysis. CCDC-652571 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif..