Nanomaterials for Rechargeable Lithium Batteries†
Peter G. Bruce Prof.
School of Chemistry, University of St. Andrews, St. Andrews, Fife, KY16 9ST, UK, Fax: (+44) 1334-463808
Search for more papers by this authorBruno Scrosati Prof.
Dipartimento di Chimica, Università di Roma, Rome, Italy
Search for more papers by this authorJean-Marie Tarascon Prof.
Laboratoire de Reactivite et de Chimie des Solides, Universite de Picardie, Amiens, France
Search for more papers by this authorPeter G. Bruce Prof.
School of Chemistry, University of St. Andrews, St. Andrews, Fife, KY16 9ST, UK, Fax: (+44) 1334-463808
Search for more papers by this authorBruno Scrosati Prof.
Dipartimento di Chimica, Università di Roma, Rome, Italy
Search for more papers by this authorJean-Marie Tarascon Prof.
Laboratoire de Reactivite et de Chimie des Solides, Universite de Picardie, Amiens, France
Search for more papers by this authorThanks to Dr. Aurelie Debart for preparation of the frontispiece.
Graphical Abstract
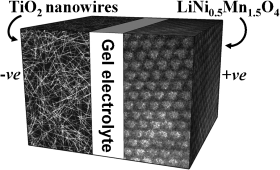
Rechargeable lithium batteries have developed into the dominant energy source for portable electronic devices because of their high energy density. Nanomaterials are pivotal for further development in this area, as their special properties can greatly increase the efficiency of such batteries. The picture shows a lithium nanobattery incorporating a TiO2(B) nanowire anode and nanoparticulate Li(Ni1/2Mn3/2)O4 cathode.
Abstract
Energy storage is more important today than at any time in human history. Future generations of rechargeable lithium batteries are required to power portable electronic devices (cellphones, laptop computers etc.), store electricity from renewable sources, and as a vital component in new hybrid electric vehicles. To achieve the increase in energy and power density essential to meet the future challenges of energy storage, new materials chemistry, and especially new nanomaterials chemistry, is essential. We must find ways of synthesizing new nanomaterials with new properties or combinations of properties, for use as electrodes and electrolytes in lithium batteries. Herein we review some of the recent scientific advances in nanomaterials, and especially in nanostructured materials, for rechargeable lithium-ion batteries.
References
- 1 Advances in Lithium-ion Batteries (Eds.: ), Kluwer Academic/Plenum, New York, 2002.
- 2 Lithium Batteries Science and Technology (Eds.: ), Kluwer Academic/Plenum, Boston, 2004.
- 3M. S. Whittingham, Chem. Rev. 2004, 104, 4271.
- 4A. S. Aricò, P. Bruce, B. Scrosati, J.-M. Tarascon, W. Van Schalkwijc, Nat. Mater. 2005, 4, 366.
- 5F. Jiao, P. G. Bruce, Adv. Mater. 2007, 19, 657.
- 6P. Balaya, A. J. Bhattacharyya, J. Jamnik, Y. F. Zhukovskii, E. A. Kotomin, J. Maier, J. Power Sources 2006, 159, 171.
- 7N. Meethong, H.-Y. S. Huang, W. C. Carter, Y.-M. Chiang, Electrochem. Solid-State Lett. 2007, 10, A 134.
- 8E. Peled, J. Electrochem. Soc. 1979, 126, 40.
- 9R. Fong, V. von Schen, J. R. Aohn, J. Electrochem. Soc. 1990, 137, 2009.
- 10R. Hanno, Y. Kawamdo, J. Electrochem. Soc. 1992, 139, 3397.
- 11R. S. Morris, B. G. Dixon, T. Gennett, R. Raffaelle, M. J. Heben, J. Power Sources 2004, 138, 277.
- 12Z. Zhou, J. J. Zhao, X. P. Gao, Z. F. Chen, J. Yan, P. V. Schiever, M. Morinaga, Chem. Mater. 2005, 17, 992.
- 13E. Ferg, R. J. Gummow, A. Dekock, M. M. Thackeray, J. Electrochem. Soc. 1994, 141, L147.
- 14A. N. Jansen, A. J. Kahalan, K. D. Kepler, P. A. Nelson, K. Amine, D. W. Dees, D. R. Vissers, M. M. Thackeray, J. Power Sources 1999, 81, 902.
- 15P. Reale, S. Panero, B. Scrosati, J. Garche, M. Wohlfahrt-Mehrens, M. Wachtler, J. Electrochem. Soc. 2004, 151, A 2138.
- 16A. R. Armstrong, G. Armstrong, J. Canales, P. G. Bruce, Angew. Chem. 2004, 116, 2336;
10.1002/ange.200353571 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 2286.
- 17A. R. Armstrong, G. Armstrong, J. Canales, R. Garcia, P. G. Bruce, Adv. Mater. 2005, 17, 862.
- 18A. R. Armstrong, G. Armstrong, J. Canales, P. G. Bruce, Chem. Commun. 2005, 2454.
- 19G. Armstrong, A. R. Armstrong, P. G. Bruce, P. Reale, B. Scrosati, Adv. Mater., 2006, 18, 2597.
- 20M.-S. Park, G.-X. Wang, Y.-M. Kang, D. Wexler, S.-X. Dou, H.-K. Liu, Angew. Chem. 2007, 119, 764; Angew. Chem. Int. Ed. 2007, 46, 750.
- 21C. K. Chan, H. L. Peng, R. D. Twesten, K. Jarausch, X. F. Zhang, Y. Cui, Nano Lett. 2007, 7, 490.
- 22K. T. Nam, D.-W. Kim, P. J. Yoo, C.-Y. Chiang, N. Meethong, P. T. Hammond, Y.-M. Chiang, A. M. Belcher, Science 2006, 312, 885.
- 23M. Winter, J. O. Besenhard, Electrochim. Acta 1999, 45, 31.
- 24R. A. Huggins in Lithium Batteries (Eds.: ), Kuwer Academic, Boston, 2004, p. 270.
- 25H. Mukaibo, T. Osaka, P. Reale, S. Panero, B. Scrosati, M. Wacthler, J. Power Sources 2004, 132, 225.
- 26I. Amadei, S. Panero, B. Scrosati, G. Cocco, L. Schiffini, J. Power Sources 2005, 143, 227.
- 27H. Mukaibo, T. Sumi, T. Yokoshima, T. Momma, T. Osaka, Electrochem. Solid-State Lett. 2003, 6, A 218.
- 28J. Hassoun, S. Panero, B. Scrosati, J. Power Sources 2006, 160, 1336.
- 29M. Green, E. Fielder, B. Scrosati, M. Wachtler, J. Serra Moreno, Electrochem. Solid-State Lett. 2003, 6, A 75.
- 30A. Timons, J. Li, J. Dahn, IMLB 2006, Biarritz, France, June 18–23, 2006, Abstract 229.
- 31P. L. Taberna, S. Mitra, P. Piozot, P. Simon, J. M. Tarascon, Nat. Mater. 2006, 5, 567.
- 32aJ. Hassoun, S. Panero, P. Simon, P. L. Taberna, B. Scrosati, Adv. Mater. 2007, 19, 1632;
- 32bA. Timmons, J. R. Dahn, J. Electrochem. Soc. 2007, 154, A 444.
- 33K. Nakajima, Batteries 2006, 6–8th June, Paris, France.
- 34H. Inoue, IMLB 2006, Biarritz, France, June 18–23, 2006, Abstract 228.
- 35K. D. Kepler, J. T. Vaughey, M. M. Thackeray, Electrochem. Solid-State Lett. 1999, 2, 307.
- 36
- 36aM. M. Thackeray, Nat. Mater. 2002, 1, 81;
- 36bL. M. L. Fransson, J. T. Vaughey, R. Benedek, K. Edstrom, J. O. Thomas, M. M. Thackeray, Electrochem. Commun. 2001, 3, 317.
- 37J. Thomas, Nat. Mater. 2003, 2, 201.
- 38N. Ravet, J. B. Goodenough, S. Besner, M. Simoneau, P. Hovington, M. Armand, Improved iron-based cathode material, ECS Fall meeting, Hawai Abstract N°127, 1999.
- 39M. Morcrette, P. Rozier, L. Dupont, E. Mugnier, L. Sannier, J. Galy, J. M. Tarascon, Nat. Mater. 2003, 2, 755.
- 40P. Poizot, S. Laruelle, S. Grugeon, L. Dupont, J.-M. Tarascon, Nature 2000, 407, 496.
- 41P. Poizot, S. Laruelle, S. Grugeon, L. Dupont, B. Beaudoin, J.-M. Tarascon, C. R. Acad. Sci. Ser. IIc 2000, 3, 681.
- 42N. Pereira, L. Dupont, J. M. Tarascon, L. C. Klein, G. G. Amatucci, J. Electrochem. Soc. 2003, 150, A 1273.
- 43H. Li, G. Richter, J. Maier, Adv. Mater. 2003, 15, 736.
- 44F. Badway, N. Pereira, F. Cosandey, G. G. Amatucci, J. Electrochem. Soc. 2003, 150, A 1209.
- 45B. Bervas, F. Badway, L. C. Klein, G. G. Amatucci, Electrochem. Solid-State Lett. 2005, 8, A 179.
- 46D. C. C. Silva, O. Crosnier, G. Ouvrard, J. Greedan, A. Safa-Sefat, L. Nazar, Electrochem. Solid-State Lett. 2003, 6, A 162.
- 47V. Pralong, D. C. S. Souza, K. T. Leung, L. Nazar, Electrochem. Commun. 2002, 4, 516.
- 48P. Poizot, S. Laruelle, S. Grugeon, L. Dupont, J. M. Tarascon, Ionics 2000, 6, 321.
- 49P. L. Taberna, S. Mitra, P. Poizot, P. Simon, J. M. Tarascon, Nat. Mater. 2006, 7, 567.
- 50J.-M. Tarascon, S. Grugeon, S. Laruelle, D. Larcher, P. Poizot in Lithium Batteries—Science and Technology (Eds.: ), Kluwer Academic Publishers, Boston, 2003, chap. 7.
- 51S. Grugeon, S. Laruelle, L. Dupont, F. Chevallier, L. Gireaud, P. L. Taberna, P. Simon, B. Yrieix, E. Vidal, S. Lascaud, J. M. Tarascon, Chem. Mater. 2005, 17, 5041.
- 52F. Gillot, S. Boyanov, L. Dupont, M. L. Doublet, M. Morcrette, L. Monconduit, J. M. Tarascon, Chem. Mater. 2005, 17, 6327.
- 53J. Bhattacharyya, J. Maier, Adv. Mater. 2004, 16, 811.
- 54F. Grey, Solid Polymer Electrolytes, VCH, Weinheim, 1991.
- 55See ref. [1].
- 56B. Scrosati, Chem. Rec. 2005, 5( 5), 286.
- 57C. A. Vincent, B. Scrosati, Bull. Mater. Res. Soc. 2000, 25, 28.
- 58F. Grey, M. Armand in Handbook of Battery Materials (Ed.: ), Wiley-VCH, Weinheim, 1999, p. 499.
- 59 Handbook of Battery Materials (Ed.: ), Wiley-VCH, Weinheim, 1999.
- 60F. Croce, G. B. Appetecchi, L. Persi, B. Scrosati, Nature 1998, 394, 456.
- 61F. Croce, R. Curini, A. Martinelli, L. Persi, F. Ronci, B. Scrosati, J. Phys. Chem. B 1999, 103, 10632.
- 62F. Croce, B. Scrosati, Ann. N. Y. Acad. Sci. 2003, 984, 194.
- 63G. B. Appetecchi, F. Croce, L. Persi, L. Ronci, B. Scrosati, Electrochim. Acta 2000, 45, 1481.
- 64F. Croce, L. Settimi, B. Scrosati, Electrochem. Commun. 2006, 8, 364.
- 65R. J. Gillespie, Acc. Chim. Res. 1968, 1, 202.
- 66V. Bolis, G. Magnaccia, G. Cerrato, C. Morterra, Langmuir 1997, 13, 888.
- 67P. G. Bruce, J. Evans, C. A. Vincent, Solid State Ionics 1988, 28, 918.
- 68F. Croce, L. Settimi, B. Scrosati, Electrochem. Commun. 2006, 8, 364.
- 69J. B. Kerr in Lithium Batteries, Science and Technology (Eds.: ), Kluwer Academic, Dordrecht, 2003, p. 602.
- 70F. Croce, S. Sacchetti, B. Scrosati, J. Power Sources 2006, 162, 685.
- 71Z. Gadjourova, Y. Andreev, D. P. Tunstall, P. G. Bruce, Nature 2001, 412, 148.
- 72A. M. Christie, S. J. Lilley, E. Staunton, Y. G. Andreev, P. G. Bruce, Nature 2005, 433, 50.
- 73Z. Stoeva, I. Martin-Litas, E. Staunton, Y. G. Andreev, P. G. Bruce, J. Am. Chem. Soc. 2003, 125, 4619.
- 74E. Stanton, Y. G. Andreev, P. G. Bruce, Faraday Discuss. 2007, 134, 143.
- 75H. K. Liu, G. X. Wang, Z. P. Guo, J. Z. Wang, K. Konstantinov, J. Nanosci. Nanotechnol. 2006, 6, 1.
- 76S. H. Ye, J. Y. Lv, W. P. Gao, F. Wu, D. Y. Song, Electrochim. Acta 2004, 49, 162.
- 77A. K. Padhi, K. S. Nanjundaswamy, C. Masquelier, S. Okada, J. B. Goodenough, J. Electrochem. Soc. 1997, 144, 1609.
- 78V. Srinivasan, J. Newman, Electrochem. Solid-State Lett. 2006, 9, A 110.
- 79H. Huang, S-C. Yin, L. F. Nazar, Electrochem. Solid-State Lett. 2001, 4, A 170.
- 80C. Delacort, P. Poizot, S. Levasseur, C. Masquelier, Electrochem. Solid-State Lett. 2006, 9, A 352.
- 81aA. Ravet, Y. Chouinard, J. F. Magnan, S. Besner, M. Gauthiér, M. Armand, J. Power Sources 2001, 97, 503;
- 81bN. Meethong, H. Y. S. Huang, W. C. Carter, Y. M. Chiang, Electrochem. Solid-State Lett. 2007, 10, A 134.
- 82P. G. Bruce, A. R. Armstrong, R. Gitzendanner, J. Mater. Chem. 1999, 9, 193.
- 83A. D. Robertson, A. R. Armstrong, A. J. Fowkes, P. G. Bruce, J. Mater. Chem. 2001, 11, 113.
- 84A. R. Armstrong, A. J. Paterson, A. D. Robertson, P. G. Bruce, Chem. Mater. 2002, 14, 710.
- 85M. M. Thackeray, W. I. F. David, P. G. Bruce, J. B. Goodenough, Mater. Res. Bull. 1983, 18, 461.
- 86A. D. Robertson, A. R. Armstrong, P. G. Bruce, Chem. Mater. 2001, 13, 2380.
- 87Y. Shao-Horn, S. A. Hackney, A. R. Armstrong, P. G. Bruce, R. Gitzendanner, C. S. Johnson, M. M. Thackeray, J. Electrochem. Soc. 1999, 146, 2404.
- 88H. F. Wang, Y. I. Jang, Y.-M. Chiang, Electrochem. Solid-State Lett. 1999, 2, 490.
- 89S. H. Kang, J. B. Goodenough, L. K. Rabenberg, Chem. Mater. 2001, 13, 1758.
- 90S. Nordlinder, L. Nyholm, T. Gustafsson, K. Edstrom, Chem. Mater. 2006, 18, 495.
- 91
- 91aF. Jiao, K. M. Shaju, P. G. Bruce, Angew. Chem. 2005, 117, 6708; Angew. Chem. Int. Ed. 2005, 44, 6550;
- 91bF. Jiao, P. G. Bruce, submitted.
- 92Y. K. Zhou, H. L. Li, J. Mater. Chem. 2002, 12, 681.
- 93J. D. Epping, B. F. Chonelka, Curr. Opin. Colloid Interface Sci. 2006, 11, 81.
- 94K. H. Reiman, K. M. Brace, T. J. Gordon-Smith, I. Nandhakumar, G. S. Attard, J. R. Owen, Electrochem. Commun. 2006, 8, 517.
- 95P. A. Nelson, J. R. Owen, J. Electrochem. Soc. 2003, 150, A 1313.
- 96B. B. Owens, S. Passerini, W. H. Smyrl, Electrochim. Acta 1999, 45, 215.
- 97J. Kim, A. Manthiram, Electrochem. Solid-State Lett. 1998, 5, 207.
- 98P. E. Tang, J. S. Sakamoto, E. Baudrin, B. Dunn, J. Non-Cryst. Solids 2004, 350, 67.
- 99H. W. Yan, S. Sokolov, J. C. Lytle, A. Stein, F. Zhang, W. H. Smyrl, J. Electrochem. Soc. 2003, 150, A 1102.
- 100G. A. Armstrong, P. G. Bruce, personal communication 2006.
- 101J. Lee, J. Kim, J. Microelectromech. Syst. 2000, 9, 71.
- 102G. Nagasubramanian, D. H. Doughty, J. Power Sources 2004, 136, 395.
- 103R. W. Hart, H. S. White, B. Dunn, D. R. Rolison, Electrochem. Commun. 2003, 5, 120.
- 104C. L. Wang, L. Taherabadi, G. Y. Jia, M. Madou, Y. T. Yeh, B. Dunn, Electrochem. Solid-State Lett. 2004, 7, A 435–A438.
- 105B. Dunn, Chem. Rev. 2003, 104, 4463.
- 106A. Singh, J. Jarayan, M. Madou, S. Akhbar, J. Electrochem. Soc. 2002, 149, E 78.
- 107M. Nathan, D. Haronian, E. Peled, US Patent No. 6,197,450, 2004.
- 108M. Nathan, D. Golodnisky, V. Yufit, E. Strauss, T. Ripenbein, I. Shechtman, S. Menkin, E. Peled, J. MELS 2005, 14, 879.
- 109Y. K. Cho, R. Wartena, S. M. Tobias, Y.-M. Chiang, Adv. Funct. Mater. 2007, 17, 379.
- 110P. L. Taberna, P. Simon, J. M. Tarascon, French Patent No. 0,504,960, 2006.
- 111Y. Shao, G. Yin, Z. Wang, Y. Gao, J. Power Sources 2007, 167, 235.