Generation of a RuII–Semiquinone–Anilino-Radical Complex through the Deprotonation of a RuIII–Semiquinone–Anilido Complex†
We thank Prof. T. Yokoyama and Dr. T. Nakagawa at the Institute for Molecular Science for measuring the X-ray photoelectron spectra (XPS) and the Research Center for Molecular-Scale Nanoscience at the Institute for Molecular Science for measurements with the SQUID magnetometer. This research was supported financially by a Grant-in-Aid for Scientific Research on Priority Areas (No. 18033057). Research carried out at the Brookhaven National Laboratory was funded by the Division of Chemical Sciences, Geosciences, & Biosciences, Office of Basic Energy Sciences of the U.S. Department of Energy under the contract DE-AC02-98CH10886.
Graphical Abstract
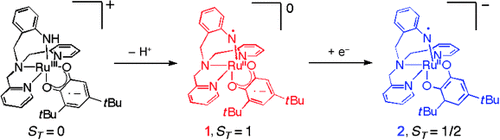
Calming the radicals: Aminyl radicals may one day be useful oxidation catalysts if their thermodynamic instability can be overcome, for example, through complexation with a metal. The semiquinone–anilino-radical and catechol–anilino-radical complexes 1 and 2 have now been prepared and the anilino-radical character of the tetradentate amine ligand proved by electron paramagentic resonance and resonance Raman spectroscopy, as well as DFT calculations.