Bis(1,2,3,4-η4-anthracene)ferrate(1−): A Paramagnetic Homoleptic Polyarene Transition-Metal Anion†
William W. Brennessel
Department of Chemistry, University of Minnesota, Minneapolis, MN 55455, USA, Fax: (+1) 612-626-7541
Current address: Department of Chemistry, University of Rochester, Rochester, NY 14627, USA
Search for more papers by this authorRobert E. Jilek
Department of Chemistry, University of Minnesota, Minneapolis, MN 55455, USA, Fax: (+1) 612-626-7541
Search for more papers by this authorJohn E. Ellis Prof.
Department of Chemistry, University of Minnesota, Minneapolis, MN 55455, USA, Fax: (+1) 612-626-7541
Search for more papers by this authorWilliam W. Brennessel
Department of Chemistry, University of Minnesota, Minneapolis, MN 55455, USA, Fax: (+1) 612-626-7541
Current address: Department of Chemistry, University of Rochester, Rochester, NY 14627, USA
Search for more papers by this authorRobert E. Jilek
Department of Chemistry, University of Minnesota, Minneapolis, MN 55455, USA, Fax: (+1) 612-626-7541
Search for more papers by this authorJohn E. Ellis Prof.
Department of Chemistry, University of Minnesota, Minneapolis, MN 55455, USA, Fax: (+1) 612-626-7541
Search for more papers by this authorHighly Reduced Organometallics, Part 62. This work was supported by the U.S. National Science Foundation and the donors of the Petroleum Research Fund, administered by the American Chemical Society. We thank Ms. Christine Lundby for expert assistance in the preparation of the manuscript. Part 61: R. E. Jilek, G. Tripepi, E. Urnezius, W. W. Brennessel, V. G. Young, Jr., J. E. Ellis, Chem. Commun. 2007, 2639.
Graphical Abstract
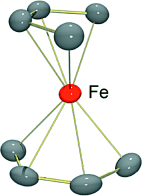
Magnetic personalities: [Fe(η4-C14H10)2]− is an unprecedented example of an anionic 17-electron homoleptic polyarene transition-metal complex. This species is the first isolable homoleptic polyarene iron complex. It provides “naked” atomic Fe−I in its reaction with 1,3-butadiene to yield the first structurally authenticated homoleptic bis(1,3-butadiene) metal complex, [Fe(η4-C4H6)2]− (see structure).
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2007/z701353_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Wilke's remarkable (trans,trans,trans-1,5,9-cyclododecatriene)nickel(0) was the first storable source of a transition-metal atom and due to its high reactivity is often called “naked nickel.” See: C. Elschenbroich, Organometallics, 3rd edition, Wiley-VCH, Weinheim, 2006, pp. 397, 694.
- 2
- 2aN. P. D. Thi, S. Spichiger, P. Paglia, G. Bernardinelli, E. Kündig, P. L. Timms, Helv. Chim. Acta 1992, 75, 2593, and references therein;
- 2bJ. A. S. Howell, N. F. Ashford, D. T. Nixon, J. C. Kola, T. A. Albright, S. K. Kang, Organometallics 1991, 10, 1852;
- 2cS. Zhang, J. K. Shen, F. Basolo, T. D. Ju, R. F. Lang, G. Kiss, C. D. Hoff, Organometallics 1994, 13, 3692;
- 2dF. Basolo, New J. Chem. 1994, 18, 19;
- 2eG. Zhu, K. E. Hanak, J. S. Figueroa, G. Parkin, J. Am. Chem. Soc. 2006, 128, 5452.
- 3
- 3a[Ru(C10H8)2]2+: E. O. Fischer, C. Elschenbroich, C. G. Kreiter, J. Organomet. Chem. 1967, 7, 481;
- 3b[M(C10H8)2], M=Cr, Mo: E. P. Kündig, P. L. Timms, J. Chem. Soc. Chem. Commun. 1977, 912;
- 3c[Cr(C10H8)2]: C. Elschenbroich, R. Möckel, Angew. Chem. 1977, 89, 908; Angew. Chem. Int. Ed. Engl. 1977, 16, 870;
- 3d[Cr(C14H10)2]: C. Elschenbroich, R. Möckel, E. Bilger, Z. Naturforsch. B 1984, 39, 375.
- 4M. Jang, J. E. Ellis, Angew. Chem. 1994, 106, 2036; Angew. Chem. Int. Ed. Engl. 1994, 33, 1973.
- 5
- 5aL=anthracene: W. W. Brennessel, V. G. Young, Jr., J. E. Ellis, Angew. Chem. 2002, 114, 1259;
10.1002/1521-3757(20020402)114:7<1259::AID-ANGE1259>3.0.CO;2-G Google ScholarAngew. Chem. Int. Ed. 2002, 41, 1211;10.1002/1521-3773(20020402)41:7<1211::AID-ANIE1211>3.0.CO;2-I CAS PubMed Web of Science® Google Scholar
- 5bL=naphthalene: W. W. Brennessel, V. G. Young, Jr., J. E. Ellis, Angew. Chem. 2006, 118, 7426;
10.1002/ange.200602937 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 7268. Isolated as a component of a “triple salt,” [K([18]c-6)]3[Co(η4-L)(C2H4)2]2[Co(η4-L)2].
- 6W. W. Brennessel, J. E. Ellis, M. K. Pomije, V. J. Sussman, E. Urnezius, V. G. Young, Jr., J. Am. Chem. Soc. 2002, 124, 10258.
- 7W. W. Brennessel, J. E. Ellis, S. N. Roush, B. R. Strandberg, O. E. Woisetschläger, V. G. Young, Jr., Chem. Commun. 2002, 2356.
- 8A claim for the existence of bis(naphthalene)iron(0) remains unverified: P. D. Morand, C. G. Francis, Inorg. Chem. 1985, 4, 1653.
- 9Only heteroleptic anthracene and naphthalene iron complexes have been previously established, for which structurally characterized examples below are designated by (X-ray):
- 9a[Fe(η4-C14H10)(CO)3]: T. A. Manuel, Inorg. Chem. 1964, 3, 1794;
- 9b[Fe(η4-C14H10)(dmpe)2], dmpe=1,2-bis(dimethylphosphano)ethane: C. A. Tolman, S. D. Ittel, A. D. English, J. P. Jesson, J. Am. Chem. Soc. 1978, 100, 4080;
- 9c[Fe2(μ-η3:η3-C14H10)(CO)6] (X-ray): M. J. Begley, S. G. Puntambekar, A. H. Wright, J. Chem. Soc. Chem. Commun. 1987, 1251;
- 9d[Fe(5–8-η4-1,4-C10H6Me2)Fe(P(OMe)3)3] (X-ray): H. Schäufele, D. Hu, H. Pritzkow, U. Zenneck, Organometallics 1989, 8, 396;
- 9e[Fe(η4-C10H8)(η6-C6Me6)] (X-ray): C. Brodt, S. Niu, H. Pritzkow, M. Stephan, U. Zenneck, J. Organometal. Chem. 1993, 459, 283.
- 9f[Fe(η6-C10H8)(dcpe)], dcpe=1,2-bis(dicyclohexylphosphano)ethane (X-ray): H. Kubo, M. Hirano, S. Komiya, J. Organomet. Chem. 1998, 556, 89.
- 10J. E. Ellis, Inorg. Chem. 2006, 45, 3167; see Table 2.
- 11Corresponding reductions of FeBr2 by alkali-metal naphthalenes provided deep red solutions of presently unknown products. W. W. Brennessel, J. E. Ellis, unpublished results.
- 12C. Elschenbroich, Organometallics, 3rd ed., Wiley-VCH, Weinheim, 2006, p. 491.
- 13Interatomic data for anion 1 b have slightly smaller uncertainties (esd's) than those for 1 a, so only the former will be discussed in this article, as anion 1.
- 14
- 14aCrystal data for 1 a: C48H60FeKO8, Mr=859.91, monoclinic, space group Pn, brown-black block, a=10.7739(6), b=9.3435(5), c=22.262(1) Å, β=97.189(1)°, V=2223.4(2) Å3, Z=2, T=173(2) K, λ=0.71073 Å, 11 569 reflections, 6340 independent, R1=0.0407 (I>2σ(I)), wR2=0.0850 (all data), μ=0.485 mm−1 (SADABS), full-matrix least-squares refinement on F2;
- 14b1 b: C48H60FeKN2O6.5, Mr=863.93, monoclinic, space group C2/c, red-green plate, a=22.763(3), b=11.271(1), c=36.390(4) Å, β=108.102(2)°, V=8874(2) Å3, Z=8, T=173(2) K, λ=0.71073 Å, 36 509 reflections, 7829 independent, R1=0.0399 (I>2σ(I)), wR2=0.0988 (all data), μ=0.485 mm−1 (SADABS), full-matrix least-squares refinement on F2;
- 14cCCDC-637186 (1 a), CCDC-637187 (1 b), CCDC-637188 (4), CCDC-637189 (5), and CCDC-637190 (6) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 15Coordination geometry is determined by a twist angle, θ, corresponding to the intersection of planes defined by the midpoints of the outer CC bonds of the coordinated dienes and the iron center. For 1, θ=85°, compared to 90° for a tetrahedral geometry. In the case of the cod complex 5, the midpoints of the olefinic CC bonds were used in the calculation of θ.
- 16Average outer C1C2 and inner C2C3 bond lengths in 1 are 1.417(5) and 1.407(4), whereas corresponding values in 2 are 1.416(8) and 1.420(6). A definite, though weak, long-short-long pattern in the coordinated diene CC bond lengths of 1, but not 2, suggests that Fe−I may back-bond to anthracene slightly better than Co−I does in these compounds. However, the metal–carbon bond lengths in 1 and 2 do not support this view and are statistically identical. Thus, the average MC1,C4 and MC2,C3 bond lengths in 1 (M=Fe) are 2.14(1) and 2.02(1) Å, respectively, and corresponding values in 2 (M=Co) are 2.13(1) and 1.99(2) Å, where the difference in atomic radii of iron and cobalt is only about 0.01 Å. See: J. Emsley, The Elements, 3rd edition, Oxford, New York, 1998, pp. 60, 106. Also, the average fold angles for coordinated anthracenes in 1 and 2 are 24 and 28°, respectively.
- 17
- 17aH. B. Chin, M. B. Smith, R. D. Wilson, R. Bau, J. Am. Chem. Soc. 1974, 96, 5285;
- 17bJ.-J. Brunet, D. Neibecker, R. S. Srivastava, J. Organomet. Chem. 1993, 461, 169;
- 17cB. Neumüller, W. Petz, Organometallics 2001, 20, 163, and references therein.
- 18
- 18aP. S. Skell, E. M. Van Dam, M. P. Silva, J. Am. Chem. Soc. 1974, 96, 626;
- 18bP. S. Skell, M. J. McGlinchey, Angew. Chem. 1975, 87, 215; Angew. Chem. Int. Ed. Engl. 1975, 14, 195;
- 18cW. Gausing, G. Wilke, Angew. Chem. 1981, 93, 201; Angew. Chem. Int. Ed. Engl. 1981, 20, 186;
- 18dM. Kaupp, T. Kopf, A. Murso, D. Stalke, C. Strohmann, J. R. Hanks, F. G. N. Cloke, P. B. Hitchcock, Organometallics 2002, 21, 5021.
- 19
- 19aP. W. Jolly, R. Mynott, Adv. Organomet. Chem. 1981, 19, 257;
- 19bK. Jonas, Adv. Organomet. Chem. 1981, 19, 97.
- 20Crystal data for 4: C26H48FeKN2O6, Mr=579.61, monoclinic, space group P21/c, red-orange block, a=10.225(1), b=28.679(3), c=10.167(1) Å, β=91.662(2)°, V=2980.0(6) Å3, Z=4, T=173(2) K, λ=0.71073 Å, 34 928 reflections, 6838 independent, R1=0.0395 (I>2σ(I)), wR2=0.0759 (all data), μ=0.685 mm−1 (SADABS), full-matrix least-squares refinement on F2. See reference [14] for CCDC number and related information.
- 21For example, the average outer and inner FeC distances in 4, 2.08(2) and 2.02(2), respectively, are essentially identical to corresponding values observed for the 18-electron Fe0 complex [Fe(η4-C4H6)2(PMe3)] (2.084(4) and 2.021(4) Å).[22a] Outer FeC distances in substituted {Fe(η4-1,3-diene)} complexes tend to be longer, presumably because of steric effects.[22b] Thus, the average outer FeC distance in 1 is 2.14(1) Å, whereas respective average inner FeC and coordinated diene CC distances in 1 and 4 are nearly the same.
- 22
- 22aJ. M. McCall, J. R. Morton, Y. Le Page, K. F. Preston, Organometallics 1984, 3, 1299;
- 22bF. A. Cotton, V. W. Day, B. A. Frenz, K. I. Hardcastle, J. M. Troup, J. Am. Chem. Soc. 1973, 95, 4522.
- 23Thus, the MC distances in 4 have a distinct long-short-short-long pattern, characteristic of predominant diolefin coordination, whereas those in [Mo(η4-C4H6)3] have a definite short-long-long-short pattern, which is indicative of a substantial metallacyclopentene contribution to the metal–butadiene bonding.[18d, 24]
- 24M. Bochmann, Organometallics 2. Complexes with Transition Metal-Carbon π-Bonds, Oxford University Press, Oxford, 1994, pp. 15–17.
- 25F. G. N. Cloke, P. B. Hitchcock, A. McCamley, J. Chem. Soc. Chem. Commun. 1993, 248. However, the presence of bulky tert-butyl groups on the 1,4-diene carbon atoms of the cobaltate causes its average outer MC distance, 2.15(2) Å, to be significantly longer than that of 4.[21]
- 26K. Jonas, L. Schieferstein, C. Krüger, Y.-H. Tsay, Angew. Chem. 1979, 91, 590;
10.1002/ange.19790910732 Google ScholarAngew. Chem. Int. Ed. Engl. 1979, 18, 550.
- 27K. Jonas, C. Krüger, Angew. Chem. 1980, 92, 513; Angew. Chem. Int. Ed. Engl. 1980, 19, 520.
- 28Several neutral 17-electron organoiron(I) complexes have been reported. See: K. Jonas, P. Klusmann, R. Goddard, Z. Naturforsch. B 1995, 50, 394.
- 29Crystal structure determinations were carried out for both salts, 5 a and 5 b, and confirmed the presence of identical anions. However, the [K([2.2.2]cryptand)]+ structure solution is of higher quality and will be reported herein. Crystal data for 5 b: C40H58FeKN2O6, Mr=757.83, triclinic, space group P
 , iridescent red-violet plate, a=12.132(5), b=13.171(6), c=14.490(7) Å, α=108.412(7), β=107.101(7), γ=107.176(7)°, V=1897.5(15) Å3, Z=2, T=173(2) K, λ=0.71073 Å, 18 644 reflections, 6696 independent, R1=0.0377 (I>2σ(I)), wR2=0.0779 (all data), μ=0.555 mm−1 (SADABS), full-matrix least-squares refinement on F2. See reference [14] for CCDC number and related information.
, iridescent red-violet plate, a=12.132(5), b=13.171(6), c=14.490(7) Å, α=108.412(7), β=107.101(7), γ=107.176(7)°, V=1897.5(15) Å3, Z=2, T=173(2) K, λ=0.71073 Å, 18 644 reflections, 6696 independent, R1=0.0377 (I>2σ(I)), wR2=0.0779 (all data), μ=0.555 mm−1 (SADABS), full-matrix least-squares refinement on F2. See reference [14] for CCDC number and related information.
- 30Average MC1,C4 and MC2,C3 bond lengths for the η4-polyarene group in 5 are 2.16(2) and 2.08(1) Å, respectively, and the corresponding values for the cobaltate are 2.15(1) and 2.010(2) Å. The average outer C1C2 and inner C2C3 bond lengths in 5 are 1.422(3) and 1.401(4), whereas corresponding values for the cobaltate are 1.419(5) and 1.400(2) Å, respectively. Average MC bond lengths for the cod groups in 5 and the cobaltate are 2.05(1) and 2.019(8) Å, respectively, whereas corresponding average olefinic CC bond lengths are 1.416(4) and 1.406(6) Å.
- 31
- 31aH. Bock, C. Arad, C. Näther, Z. Havlas, J. Chem. Soc. Chem. Commun. 1995, 2393;
- 31bH. Bock, K. Gharagozloo-Habmann, M. Slevert, T. Prisner, Z. Havlas, Nature 2000, 404, 267.
- 32Crystal data for 6: C23H37FeO9P3, Mr=606.29, monoclinic, space group P21/c, orange prism, a=9.891(2), b=17.025(4), c=16.398(4) Å, β=93.411(4)°, V=2756(1) Å3, Z=4, T=173(2) K, λ=0.71073 Å, 18 478 reflections, 6307 independent, R1=0.0340 (I>2σ(I)), wR2=0.0974 (all data), μ=0.769 mm−1 (SADABS), full-matrix least-squares refinement on F2. See reference [14] for CCDC number and related information.
- 33See the Supporting Information for the molecular structure of 6, selected interatomic data, and comparisons with [Fe(5–8-η4-1,4-dimethylnaphthalene)(P(OMe)3)3].[9d]
- 34W. W. Brennessel, J. E. Ellis, unpublished results.
- 35K. Meerholz, J. Heinze, J. Am. Chem. Soc. 1989, 111, 2325.
- 36This observation is consistent with an analysis of loss of resonance energies (ΔRE) that occur when arenes bind to a given metal, oxidation state, and ligand set.[2c] On this basis, the {M(η4-arene)} bond energy will increase in the order naphthalene<anthracene. However, the corresponding {M(η6-arene)} bond energy will increase in the opposite order, that is, anthracene<naphthalene. This simple analysis does not include steric effects or intrinsic differences in the donor and acceptor abilities of the bound arenes. A recent study by Parkin and co-workers also considers these issues.[2e]
- 37E. P. Kündig, C. Perret, S. Spichiger, G. Bernardinelli, J. Organomet. Chem. 1985, 286, 183.
- 38See:
- 38aB. de Bruin, E. Bill, E. Bothe, T. Weyhermüller, K. Wieghardt, Inorg. Chem. 2000, 39, 2936;
- 38bS. A. Stoian, Y. Yu, J. M. Smith, P. L. Holland, E. L. Bominaar, E. Münck, Inorg. Chem. 2005, 44, 4915;
- 38cM. P. Hendrich, W. Gunderson, R. K. Behan, M. T. Green, M. P. Mehn, T. A. Betley, C. C. Lu, J. C. Peters, Proc. Natl. Acad. Sci. USA 2006, 103, 17107, and references therein.