Molecular Wire Type Behavior of Polycationic Multinuclear Rack-Type RuII Complexes of Polytopic Hydrazone-Based Ligands†
Frédérique Loiseau Dr.
Dipartimento di Chimica Inorganica, Chimica Analitica e Chimica Fisica, Università di Messina, Via Sperone 31, 98166 Messina, Italy, Fax: (+39) 090-393-756
Search for more papers by this authorFrancesco Nastasi
Dipartimento di Chimica Inorganica, Chimica Analitica e Chimica Fisica, Università di Messina, Via Sperone 31, 98166 Messina, Italy, Fax: (+39) 090-393-756
Search for more papers by this authorAdrian-Mihail Stadler Dr.
ISIS—Universite Louis Pasteur, 8 Allee Gaspard Monge, BP 70028, 67083 Strasbourg cedex, France, Fax: (+33) 390-245-140 http://www-isis.u-strasbg.fr
Search for more papers by this authorSebastiano Campagna Prof. Dr.
Dipartimento di Chimica Inorganica, Chimica Analitica e Chimica Fisica, Università di Messina, Via Sperone 31, 98166 Messina, Italy, Fax: (+39) 090-393-756
Search for more papers by this authorJean-Marie Lehn Prof. Dr.
ISIS—Universite Louis Pasteur, 8 Allee Gaspard Monge, BP 70028, 67083 Strasbourg cedex, France, Fax: (+33) 390-245-140 http://www-isis.u-strasbg.fr
Search for more papers by this authorFrédérique Loiseau Dr.
Dipartimento di Chimica Inorganica, Chimica Analitica e Chimica Fisica, Università di Messina, Via Sperone 31, 98166 Messina, Italy, Fax: (+39) 090-393-756
Search for more papers by this authorFrancesco Nastasi
Dipartimento di Chimica Inorganica, Chimica Analitica e Chimica Fisica, Università di Messina, Via Sperone 31, 98166 Messina, Italy, Fax: (+39) 090-393-756
Search for more papers by this authorAdrian-Mihail Stadler Dr.
ISIS—Universite Louis Pasteur, 8 Allee Gaspard Monge, BP 70028, 67083 Strasbourg cedex, France, Fax: (+33) 390-245-140 http://www-isis.u-strasbg.fr
Search for more papers by this authorSebastiano Campagna Prof. Dr.
Dipartimento di Chimica Inorganica, Chimica Analitica e Chimica Fisica, Università di Messina, Via Sperone 31, 98166 Messina, Italy, Fax: (+39) 090-393-756
Search for more papers by this authorJean-Marie Lehn Prof. Dr.
ISIS—Universite Louis Pasteur, 8 Allee Gaspard Monge, BP 70028, 67083 Strasbourg cedex, France, Fax: (+33) 390-245-140 http://www-isis.u-strasbg.fr
Search for more papers by this authorThe University of Messina and PRIN are acknowledged for financial support.
Graphical Abstract
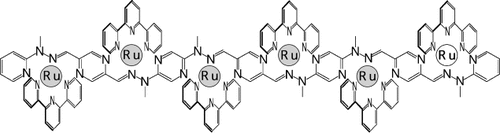
On the rack: Splitting of the peripheral metal-based oxidation, which indicates a large coupling between the redox sites, has been obtained in polymetallic arrays composed of rack-type complexes containing two, three, four, or six RuII subunits appended to hydrazone-based molecular strands (see picture). The relatively small coupling attenuation parameter calculated for the metal-based, cationic subunit(s) suggests molecular-wire behavior.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2007/z700388_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aD. S. Lawrence, T. Jiang, M. Levett, Chem. Rev. 1995, 95, 2229;
- 1bD. L. Caulder, K. N. Raymond, Acc. Chem. Res. 1999, 32, 975;
- 1cG. F. Swiegers, T. J. Malefetse, Chem. Rev. 2000, 100, 3483;
- 1dS. R. Seidel, P. J. Stang, Acc. Chem. Res. 2002, 35, 972;
- 1eW.-Y. Sun, M. Yoshizawa, T. Kusukawa, M. Fujita, Curr. Opin. Chem. Biol. 2002, 6, 757;
- 1fM. D. Ward, Annu. Rep. Prog. Chem. Sect. A 2002, 98, 285;
- 1gM. Ruben, J. Rojo, F. J. Romero-Salguero, L. H. Uppadine, J.-M. Lehn, Angew. Chem. 2004, 116, 3728;
10.1002/ange.200300636 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 3644;
- 1hP. Ceroni, G. Bergamini, F. Marchioni, V. Balzani, Prog. Polym. Sci. 2005, 30, 453.
- 2
- 2aT. J. Meyer, Pure Appl. Chem. 1986, 58, 1193;
- 2bA. Juris, V. Balzani, F. Barigelletti, S. Campagna, P. Belser, A. von Zelewsky, Coord. Chem. Rev. 1988, 84, 85.
- 3For some examples, see
- 3aV. Balzani, A. Juris, M. Venturi, S. Campagna, S. Serroni, Chem. Rev. 1996, 96, 759;
- 3bJ. H. Alstrum-Acevedo, M. K. Brennaman, T. J. Meyer, Inorg. Chem. 2005, 44, 6802;
- 3cV. Balzani, A. Credi, M. Venturi, Molecular Devices and Machines, Wiley-VCH, Weinheim, 2003.
10.1002/3527601600 Google Scholar
- 4
- 4aG. S. Hanan, C. Arana, J.-M. Lehn, D. Fenske Angew. Chem. 1995, 107, 1191;
10.1002/ange.19951071010 Google ScholarAngew. Chem. Int. Ed. Engl. 1995, 34, 1122;
- 4bG. S. Hanan, C. Arana, J.-M. Lehn, G. Baum, D. Fenske, Chem. Eur. J. 1996, 2, 1292;
- 4cA.-M. Stadler, F. Puntoriero, S. Campagna, N. Kyritsakas, R. Welter, J.-M. Lehn, Chem. Eur. J. 2005, 11, 3997.
- 5For recent reviews on molecular wires, see
- 5aM. N. Paddon-Row in Electron Transfer in Chemistry, Vol. 3 (Ed.: ), 2001, p. 179, and references therein;
- 5bF. Scandola, C. Chiorboli, M. T. Indelli, M. A. Rampi in Electron Transfer in Chemistry, Vol. 3 (Ed.: ), 2001, p. 337;
10.1002/9783527618248.ch40 Google Scholar
- 5cMolecular Wires: From Design to Perspectives: Top. Curr. Chem. 2005, 257 (Ed.: L. De Cola);
- 5dJ. L. Segura, N. Martin, D. M. Guldi, Chem. Soc. Rev. 2005, 34, 31, and references therein.
- 6Strictly speaking, molecular-wire behavior means electrical conduction through a bridge, which requires that the electron from the donor, which is thermally injected into the conduction band of the bridge, becomes localized within the bridge and is transported through the bridge, from donor to acceptor, by an incoherent scattering mechanism. For this type of system, the distance dependence of the electron transfer (and of the electronic coupling between the donor and acceptor) is ohmic, and therefore varies inversely with bridge length.[5,7] In the superexchange mechanism, the electron never resides on the bridge orbitals. However, the term “molecular wire” is commonly extended to systems operating through the superexchange mechanism as well, when electronic coupling between the peripheral active sites of the linear assembly is large enough to allow fast intercomponent electron- and/or energy-transfer processes.
- 7W. B. Davis, M. R. Wasielewsky, M. A. Ratner, V. Mujica, A. Nitzan, J. Phys. Chem. A 1997, 101, 6158.
- 8H. H. McConnell, J. Chem. Phys. 1961, 35, 508.
- 9For former attempts to correlate redox-potential splitting with spectroscopic data for determining electronic coupling, see
- 9aR. De la Rosa, P. J. Chang, F. Salaymeh, J. C. Curtis, Inorg. Chem. 1985, 24, 4229;
- 9bY. Dong, J. T. Hupp, Inorg. Chem. 1992, 31, 3170;
- 9cC. E. B. Evans, M. L. Naklicki, A. R. Rezvani, C. A. White, V. V. Kondratiev, R. J. Crutchley, J. Am. Chem. Soc. 1998, 120, 13096;
- 9dC. Lambert, G. Nöll, J. Am. Chem. Soc. 1999, 121, 8434.
- 10
- 10aM. B. Robin, P. Day, Adv. Inorg. Chem. Radiochem. 1967, 10, 247;
- 10bD. E. Richardson, H. Taube, J. Am. Chem. Soc. 1983, 105, 40;
- 10cC. Creutz, Prog. Inorg. Chem. 1983, 30, 1.
- 11
- 11aR. J. Crutchley, Adv. Inorg. Chem. 1994, 41, 273;
- 11bG. Giuffrida, S. Campagna, Coord. Chem. Rev. 1994, 135–136, 517;
- 11cJ.-P. Launay, Chem. Soc. Rev. 2001, 30, 386, and references therein.
- 12M. Venturi, S. Serroni, A. Juris, S. Campagna, V. Balzani, Top. Curr. Chem. 1998, 197, 193.
- 13
- 13aL. H. Uppadine, J.-P. Gisselbrecht, J.-M. Lehn, Chem. Commun. 2004, 718;
- 13bL. H. Uppadine, J.-P. Gisselbrecht, N. Kyritsakas, K. Nättinen, K. Rissanen, J.-M. Lehn, Chem. Eur. J. 2005, 11, 2549.
- 14A. Kirsch-De Mesmaeker, L. Jacquet, A. Masschelein, F. Vanhecke, K. Heremans, Inorg. Chem. 1989, 28, 2465.
- 15Redox splitting is related in a straightforward manner to intercomponent electronic coupling only for weakly coupled systems, that is, when valence is vibrationally trapped (classes I and II of the Robin–Day mixed-valence systems).[10] Before discussing our data, it was necessary to clarify that our systems belong to this case. A detailed discussion on this point is contained in the Supporting Information.
- 16Redox splitting can also arise from a number of contributions (for example, electrostatic) besides electronic coupling.[10] Meaningful β values can be obtained from redox splittings when those additional contributions are negligible. This is the case for the present systems.
- 17
- 17aN. S. Hush, Prog. Inorg. Chem. 1967, 8, 391;
- 17bN. S. Hush, Coord. Chem. Rev. 1985, 64, 135.
- 18C. Chiorboli, M. T. Indelli, F. Scandola, Top. Curr. Chem. 2005, 257, 63, and references therein.
- 19Although β is usually considered a “bridge-specific” parameter, its value for a specific spacer (or spacer subunit) can vary on changing the peripheral subunits it connects. In fact the value of β depends on Δ, the energy gap between donor and bridge orbitals, and on T, the interaction between two adjacent, identical bridge subunits, according to the equation β=2In|(Δ/T)|.[5a,8] While T can be considered invariant, Δ depends on the whole system, including the peripheral sites.
- 20M. J. Shephard, M. N. Paddon-Row, K. D. Jordan, Chem. Phys. 1993, 176, 289.
- 21B. Schlicke, P. Belser, L. De Cola, E. Sabbioni, V. Balzani, J. Am. Chem. Soc. 1999, 121, 4207.
- 22S. F. Nelsen, H. Q. Tran, M. A. Nagy, J. Am. Chem. Soc. 1998, 120, 298.