A Novel Reaction of the “Huisgen Zwitterion” with Chalcones and Dienones: An Efficient Strategy for the Synthesis of Pyrazoline and Pyrazolopyridazine Derivatives†
Financial assistance from the Council of Scientific and Industrial Research (CSIR), New Delhi is acknowledged. The authors thank Soumini Mathew and Sreemathi Viji for recording the NMR and mass spectra, respectively.
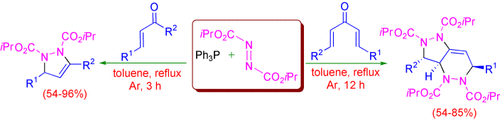
Graphical Abstract
Two unexpected transformations: The reaction of the Huisgen zwitterion derived from triphenylphosphane and diisopropyl azodicarboxylate (DIAD) with chalcones affords functionalized pyrazolines. In contrast, the reaction with dienones affords pyrazolopyridazines, presumably by Diels–Alder reaction of the initially formed pyrazoline with excess DIAD (see scheme).