Highly Asymmetric Michael Addition to α,β-Unsaturated Ketones Catalyzed by 9-Amino-9-deoxyepiquinine†
Correction(s) for this article
-
Highly Asymmetric Michael Addition to α,β-Unsaturated Ketones Catalyzed by 9-Amino-9-deoxyepiquinine
- Jian-Wu Xie,
- Wei Chen,
- Rui Li,
- Mi Zeng,
- Wei Du,
- Lei Yue,
- Ying-Chen Chen Prof. Dr.,
- Yong Wu Prof. Dr.,
- Jin Zhu Prof.,
- Jin-Gen Deng Prof. Dr.,
- Volume 46Issue 27Angewandte Chemie International Edition
- pages: 5049-5049
- First Published online: June 22, 2007
Jian-Wu Xie
Key Laboratory of Asymmetric Synthesis & Chirotechnology of Sichuan Province, Union Laboratory of Asymmetric Synthesis, Chengdu Institute of Organic Chemistry, Chinese Academy of Sciences, Chengdu, 610041, P.R. China, Fax: (+86) 28-852-239-78
Graduate School of the Chinese Academy of Sciences, Beijing, P.R. China
Search for more papers by this authorWei Chen
Key laboratory of Drug-Targeting of the Education Ministry, Department of Medicinal Chemistry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, P.R. China, Fax: (+86) 28-85502609
Search for more papers by this authorRui Li
Key laboratory of Drug-Targeting of the Education Ministry, Department of Medicinal Chemistry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, P.R. China, Fax: (+86) 28-85502609
Search for more papers by this authorMi Zeng
Key laboratory of Drug-Targeting of the Education Ministry, Department of Medicinal Chemistry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, P.R. China, Fax: (+86) 28-85502609
Search for more papers by this authorWei Du
Key laboratory of Drug-Targeting of the Education Ministry, Department of Medicinal Chemistry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, P.R. China, Fax: (+86) 28-85502609
Search for more papers by this authorLei Yue
Key laboratory of Drug-Targeting of the Education Ministry, Department of Medicinal Chemistry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, P.R. China, Fax: (+86) 28-85502609
Search for more papers by this authorYing-Chun Chen Prof. Dr.
Key laboratory of Drug-Targeting of the Education Ministry, Department of Medicinal Chemistry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, P.R. China, Fax: (+86) 28-85502609
Search for more papers by this authorYong Wu Prof. Dr.
Key laboratory of Drug-Targeting of the Education Ministry, Department of Medicinal Chemistry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, P.R. China, Fax: (+86) 28-85502609
Search for more papers by this authorJin Zhu Prof.
Key Laboratory of Asymmetric Synthesis & Chirotechnology of Sichuan Province, Union Laboratory of Asymmetric Synthesis, Chengdu Institute of Organic Chemistry, Chinese Academy of Sciences, Chengdu, 610041, P.R. China, Fax: (+86) 28-852-239-78
Search for more papers by this authorJin-Gen Deng Prof. Dr.
Key Laboratory of Asymmetric Synthesis & Chirotechnology of Sichuan Province, Union Laboratory of Asymmetric Synthesis, Chengdu Institute of Organic Chemistry, Chinese Academy of Sciences, Chengdu, 610041, P.R. China, Fax: (+86) 28-852-239-78
Search for more papers by this authorJian-Wu Xie
Key Laboratory of Asymmetric Synthesis & Chirotechnology of Sichuan Province, Union Laboratory of Asymmetric Synthesis, Chengdu Institute of Organic Chemistry, Chinese Academy of Sciences, Chengdu, 610041, P.R. China, Fax: (+86) 28-852-239-78
Graduate School of the Chinese Academy of Sciences, Beijing, P.R. China
Search for more papers by this authorWei Chen
Key laboratory of Drug-Targeting of the Education Ministry, Department of Medicinal Chemistry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, P.R. China, Fax: (+86) 28-85502609
Search for more papers by this authorRui Li
Key laboratory of Drug-Targeting of the Education Ministry, Department of Medicinal Chemistry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, P.R. China, Fax: (+86) 28-85502609
Search for more papers by this authorMi Zeng
Key laboratory of Drug-Targeting of the Education Ministry, Department of Medicinal Chemistry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, P.R. China, Fax: (+86) 28-85502609
Search for more papers by this authorWei Du
Key laboratory of Drug-Targeting of the Education Ministry, Department of Medicinal Chemistry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, P.R. China, Fax: (+86) 28-85502609
Search for more papers by this authorLei Yue
Key laboratory of Drug-Targeting of the Education Ministry, Department of Medicinal Chemistry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, P.R. China, Fax: (+86) 28-85502609
Search for more papers by this authorYing-Chun Chen Prof. Dr.
Key laboratory of Drug-Targeting of the Education Ministry, Department of Medicinal Chemistry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, P.R. China, Fax: (+86) 28-85502609
Search for more papers by this authorYong Wu Prof. Dr.
Key laboratory of Drug-Targeting of the Education Ministry, Department of Medicinal Chemistry, West China School of Pharmacy, Sichuan University, Chengdu, 610041, P.R. China, Fax: (+86) 28-85502609
Search for more papers by this authorJin Zhu Prof.
Key Laboratory of Asymmetric Synthesis & Chirotechnology of Sichuan Province, Union Laboratory of Asymmetric Synthesis, Chengdu Institute of Organic Chemistry, Chinese Academy of Sciences, Chengdu, 610041, P.R. China, Fax: (+86) 28-852-239-78
Search for more papers by this authorJin-Gen Deng Prof. Dr.
Key Laboratory of Asymmetric Synthesis & Chirotechnology of Sichuan Province, Union Laboratory of Asymmetric Synthesis, Chengdu Institute of Organic Chemistry, Chinese Academy of Sciences, Chengdu, 610041, P.R. China, Fax: (+86) 28-852-239-78
Search for more papers by this authorWe are grateful for the financial support of the National Natural Science Foundation of China (20502018), the Ministry of Education (NCET-05-0781), and Sichuan University.
Graphical Abstract
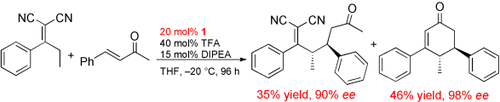
Michael–Michael–retro-Michael cascade reactions are promoted by the highly efficient organocatalyst 9-amino-9-deoxyepiquinine (1). The asymmetric direct vinylogous Michael addition of α,α-dicyanoalkenes to α,β-unsaturated ketones may be followed by an intramolecular Michael addition and a retro-Michael reaction to afford polysubstituted 2-cyclohexen-1-one derivatives with high enantioselectivity (see example).
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2007/z603612_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews, see:
- 1aP. I. Dalko, L. Moisan, Angew. Chem. 2004, 116, 5248;
10.1002/ange.200400650 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 5138;
- 1bB. List, Chem. Commun. 2006, 819;
- 1cA. Berkessel, H. Gröger, Asymmetric Organocatalysis, Wiley-VCH, Weinheim, 2005.
- 2
- 2aK. A. Ahrendt, C. J. Borths, D. W. C. MacMillan, J. Am. Chem. Soc. 2000, 122, 4243;
- 2bY. K. Chen, M. Yoshida, D. W. C. MacMillan, J. Am. Chem. Soc. 2006, 128, 9328;
- 2cJ. W. Yang, M. T. Hechavarria Fonseca, B. List, Angew. Chem. 2004, 116, 6829; Angew. Chem. Int. Ed. 2004, 43, 6660;
- 2dT. Kano, Y. Tanaka, K. Maruoka, Org. Lett. 2006, 8, 2687;
- 2eS. Brandau, A. Landa, J. Franzén, M. Marigo, K. A. Jørgensen, Angew. Chem. 2006, 118, 4411;
10.1002/ange.200601025 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 4305;
- 2fW. Chen, X.-H. Yuan, R. Li, W. Du, Y. Wu, L.-S. Ding, Y.-C. Chen, Adv. Synth. Catal. 2006, 348, 1818; for examples of primary aminocatalysts, see:
- 2gK. Ishihara, K. Nakano, J. Am. Chem. Soc. 2005, 127, 10504;
- 2hA. Sakakura, K. Suzuki, K. Nakano, K. Ishihara, Org. Lett. 2006, 8, 2229.
- 3
- 3aM. Yamaguchi, T. Shiraishi, M. Hirama, J. Org. Chem. 1996, 61, 3520;
- 3bS. Hanessian, V. Pham, Org. Lett. 2000, 2, 2975, and references therein;
- 3cA. B. Northrup, D. W. C. MacMillan, J. Am. Chem. Soc. 2002, 124, 2458;
- 3dN. Halland, P. S. Aburel, K. A. Jørgensen, Angew. Chem. 2003, 115, 685;
10.1002/ange.200390150 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 661;
- 3eN. Halland, T. Hansen, K. A. Jørgensen, Angew. Chem. 2003, 115, 5105;
10.1002/ange.200352136 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 4955;
- 3fN. Halland, P. S. Aburel, K. A. Jørgensen, Angew. Chem. 2004, 116, 1292;
10.1002/ange.200353364 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 1272;
- 3gA. Prieto, N. Halland, K. A. Jørgensen, Org. Lett. 2005, 7, 3897;
- 3hK. R. Knudsen, C. E. T. Mitchell, S. V. Ley, Chem. Commun. 2006, 66.
- 4
- 4aD. Xue, Y.-C. Chen, L.-F. Cun, Q.-W. Wang, J. Zhu, J.-G. Deng, Org. Lett. 2005, 7, 5293;
- 4bJ.-W. Xie, L. Yue, D. Xue, X.-L. Ma, Y.-C. Chen, Y. Wu, J. Zhu, J.-G. Deng, Chem. Commun. 2006, 1563; for similar studies, see:
- 4cT. B. Poulsen, M. Bell, K. A. Jørgensen, Org. Biomol. Chem. 2006, 4, 63;
- 4dT. B. Poulsen, C. Alemparte, K. A. Jørgensen, J. Am. Chem. Soc. 2005, 127, 11614.
- 5
- 5aY.-C. Chen, D. Xue, J.-G. Deng, X. Cui, J. Zhu, Y.-Z. Jiang, Tetrahedron Lett. 2004, 45, 1555;
- 5bD. Xue, Y.-C. Chen, X. Cui, Q.-W. Wang, J. Zhu, J.-G. Deng, J. Org. Chem. 2005, 70, 3584.
- 6
- 6aD. B. Ramachary, C. F. Barbas III, Chem. Eur. J. 2004, 10, 5323;
- 6bJ. W. Yang, M. T. Hechavarria Fonseca, B. List, J. Am. Chem. Soc. 2005, 127, 15036;
- 6cY. Huang, A. Walji, C. H. Larsen, D. W. C. MacMillan, J. Am. Chem. Soc. 2005, 127, 15051;
- 6dM. Marigo, T. Schulte, J. Franzén, K. A. Jørgensen, J. Am. Chem. Soc. 2005, 127, 15710;
- 6eD. Enders, M. R. M. Hüttl, C. Grondal, G. Raabe, Nature 2006, 441, 861;
- 6fM. Marigo, S. Bertelsen, A. Landa, K. A. Jørgensen, J. Am. Chem. Soc. 2006, 128, 5475;
- 6gW. Wang, H. Li, J. Wang, L. Zu, J. Am. Chem. Soc. 2006, 128, 10354;
- 6hsee also ref. 3f; for a review on tandem reactions, see:
- 6iH.-C. Guo, J.-A. Ma, Angew. Chem. 2006, 118, 362; Angew. Chem. Int. Ed. 2006, 45, 354.
- 7
- 7aS. Tsogoeva, S. B. Jagtap, Synlett 2004, 2624;
- 7bjust after the acceptance of this manuscript, the application of valine ester phosphate salts in the asymmetric transfer hydrogenation of cyclic enones was reported; see: N. J. A. Martin, B. List, J. Am. Chem. Soc. 2006, 128, 13368.
- 8Complete diastereoselectivity was also observed in other vinylogous Michael reactions of α,α-dicyanoalkenes as a result of kinetic control; see ref. [4a–c].
- 9
- 9aH. Brunner, J. Bügler, B. Nuber, Tetrahedron: Asymmetry 1995, 6, 1699;
- 9bfor a review on cinchona alkaloids as organocatalysts, see: S.-K. Tian, Y. Chen, J. Hang, L. Tang, P. McDaid, L. Deng, Acc. Chem. Res. 2004, 37, 621.
- 10CCDC-621781 (4 ac) and CCDC-621782 (4 af) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 11The chiral compound 5 ea was prepared in seven steps from acetophenone:
- 11aF. -Y, Zhang, E. J. Corey, Org. Lett. 2000, 2, 1097; for the synthesis of racemic substituted 2-cyclohexen-1-ones by metal catalysis, see:
- 11bT. Okano, Y. Satou, M. Tamura, J. Kiji, Bull. Chem. Soc. Jpn. 1997, 70, 1879.
- 12For the intermolecular Michael addition of unmodified aldehydes to vinyl ketones, see:
- 12aP. Melchiorre, K. A. Jørgensen, J. Org. Chem. 2003, 68, 4151;
- 12bT. J. Peelen, Y. Chi, S. H. Gellman, J. Am. Chem. Soc. 2005, 127, 11598;
- 12cY. Chi, S. H. Gellman, Org. Lett. 2005, 7, 4253; the Michael addition of aldehydes to β-substituted enones has only been conducted in an intramolecular fashion; see:
- 12dM. T. Hechavarria Fonseca, B. List, Angew. Chem. 2004, 116, 4048;
10.1002/ange.200460578 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 3958;
- 12eY. Hayashi, H. Gotoh, T. Tamura, H. Yamaguchi, R. Masui, M. Shoji, J. Am. Chem. Soc. 2005, 127, 16028;
- 12fsee ref. [6b].
- 13The unmodified ketones only show reactivity with highly activated alkenes in the presence of aminocatalysts; for nitroalkenes, see:
- 13aD. Enders, S. Chow, Eur. J. Org. Chem. 2006, 4578;
- 13bN. Mase, K. Watanabe, H. Yoda, K. Takabe, F. Tanaka, C. F. Barbas III, J. Am. Chem. Soc. 2006, 128, 4966;
- 13cC.-L. Cao, M.-C. Ye, X.-L. Sun, Y. Tang, Org. Lett. 2006, 8, 2901, and references therein;
- 13dY. Xu, A. Córdova, Chem. Commun. 2006, 460;
- 13eB. List, P. Pojarliev, H. Martin, Org. Lett. 2001, 3, 2423;
- 13fD. Enders, A. Seki, Synlett 2002, 26; for alkylidenemalonates, see:
- 13gJ. M. Betancort, K. Sakthivel, R. Thayumanavan, F. Tanaka, C. F. Barbas III, Synthesis 2004, 1509, and references therein.
- 14For recent reviews on the catalytic enantioselective construction of quaternary chiral with only carbon-centered substituents, see:
- 14aJ. Christoffers, A. Baro, Adv. Synth. Catal. 2005, 347, 1473;
- 14bE. A. Peterson, L. E. Overman, Proc. Natl. Acad. Sci. USA 2004, 101, 11943.
- 15The relative configuration of 6 was assigned by extensive NMR spectroscopic analysis; see Supporting Information.