Time-Controlled Microfluidic Seeding in nL-Volume Droplets To Separate Nucleation and Growth Stages of Protein Crystallization†
This work was supported by NIH Protein Structure Initiative Specialized Centers Grant GM074961 (ATCG3D) and the NIH (R01 EB001903). Use of the Advanced Photon Source was supported by the US Department of Energy (contract no. W-31–109-Eng-38). Use of the BioCARS Sector 14 was supported by the NIH National Center for Research Resources (grant number RR07707). GM/CA-CAT has been funded in whole or in part by the National Cancer Institute (Y1-CO-1020) and the National Institute of General Medical Sciences (Y1-GM-1104). Funding for functional and structural proteomics of SARS CoV-related proteins is provided through NIH-NIAID contract HHSN266200400058C. We thank Ruslan Sanishvili (GM/CA Cat station 23ID-D staff support) for technical assistance; Scott Lovell and Lance Stewart (deCODE Biostructures) for helpful assistance and discussions; Shu Moy (Midwest Center for Structural Genomics) for cloning work on Oligoendopeptidase F; Vanitha Subramanian (The Scripps Research Institute) for cloning, expression, and purification of SARS nucleocapsid N-terminal domain; and L. Spencer Roach (University of Chicago) for help with thaumatin X-ray diffraction comparisons.
Graphical Abstract
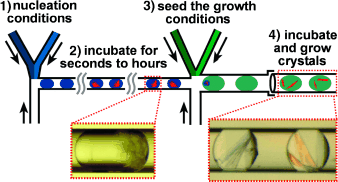
Sowing seeds: The growth of well-ordered protein crystals requires control of the two key stages of crystallization, namely, nucleation and growth. However, the ideal conditions for these two stages are often different. A microfluidic system (see picture) was used to perform time-controlled crystal seeding in nL volumes. Single crystals of the protein Oligoendopeptidase F were obtained and used to determine its X-ray crystal structure.