Total Synthesis and Structure Assignment of (+)-Hexacyclinol†
We thank Mr. Andrew Germain (Boston University) for helpful discussions, Dr. Friedrich A. Gollmick (Leibniz Institute for Natural Products Research and Infection Biology—Hans-Knöll-Institute, Jena, Germany) for providing spectra of natural hexacyclinol, and Dr. Emil Lobkovsky (Cornell University) for X-ray crystal structure analysis. Financial support from the NIH (GM62842 and P50 GM067041), Bristol-Myers Squibb, Merck, and Novartis is gratefully acknowledged.
Graphical Abstract
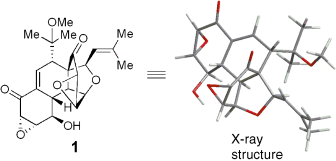
Structure assigned: The revised structure of (+)-hexacyclinol (1) proposed recently was confirmed following the total synthesis of the natural product. The synthesis was designed around the highly stereoselective Diels–Alder dimerization of an epoxyquinol monomer, followed by intramolecular acid-catalyzed cyclization.