Lydiamycins A–D: Cyclodepsipetides with Antimycobacterial Properties†
Xueshi Huang Dr.
Leibniz Institute for Natural Product Research and Infection Biology, Hans-Knöll-Institute, Beutenbergstrasse 11a, 07745 Jena, Germany, Fax: (+49) 3641-656-679
Current address: Syntheses & Biomimetic Drugs Research Center, China Medical University, Liaoning, P.R. China
Search for more papers by this authorErnst Roemer Dr.
Leibniz Institute for Natural Product Research and Infection Biology, Hans-Knöll-Institute, Beutenbergstrasse 11a, 07745 Jena, Germany, Fax: (+49) 3641-656-679
Current address: Leibniz Institute of Plant Biochemistry, Halle, Germany
Search for more papers by this authorIsabel Sattler Dr.
Leibniz Institute for Natural Product Research and Infection Biology, Hans-Knöll-Institute, Beutenbergstrasse 11a, 07745 Jena, Germany, Fax: (+49) 3641-656-679
Search for more papers by this authorUte Moellmann Dr.
Leibniz Institute for Natural Product Research and Infection Biology, Hans-Knöll-Institute, Beutenbergstrasse 11a, 07745 Jena, Germany, Fax: (+49) 3641-656-679
Search for more papers by this authorArnulf Christner Dr.
Leibniz Institute for Natural Product Research and Infection Biology, Hans-Knöll-Institute, Beutenbergstrasse 11a, 07745 Jena, Germany, Fax: (+49) 3641-656-679
Search for more papers by this authorSusanne Grabley Prof. Dr.
Leibniz Institute for Natural Product Research and Infection Biology, Hans-Knöll-Institute, Beutenbergstrasse 11a, 07745 Jena, Germany, Fax: (+49) 3641-656-679
Search for more papers by this authorXueshi Huang Dr.
Leibniz Institute for Natural Product Research and Infection Biology, Hans-Knöll-Institute, Beutenbergstrasse 11a, 07745 Jena, Germany, Fax: (+49) 3641-656-679
Current address: Syntheses & Biomimetic Drugs Research Center, China Medical University, Liaoning, P.R. China
Search for more papers by this authorErnst Roemer Dr.
Leibniz Institute for Natural Product Research and Infection Biology, Hans-Knöll-Institute, Beutenbergstrasse 11a, 07745 Jena, Germany, Fax: (+49) 3641-656-679
Current address: Leibniz Institute of Plant Biochemistry, Halle, Germany
Search for more papers by this authorIsabel Sattler Dr.
Leibniz Institute for Natural Product Research and Infection Biology, Hans-Knöll-Institute, Beutenbergstrasse 11a, 07745 Jena, Germany, Fax: (+49) 3641-656-679
Search for more papers by this authorUte Moellmann Dr.
Leibniz Institute for Natural Product Research and Infection Biology, Hans-Knöll-Institute, Beutenbergstrasse 11a, 07745 Jena, Germany, Fax: (+49) 3641-656-679
Search for more papers by this authorArnulf Christner Dr.
Leibniz Institute for Natural Product Research and Infection Biology, Hans-Knöll-Institute, Beutenbergstrasse 11a, 07745 Jena, Germany, Fax: (+49) 3641-656-679
Search for more papers by this authorSusanne Grabley Prof. Dr.
Leibniz Institute for Natural Product Research and Infection Biology, Hans-Knöll-Institute, Beutenbergstrasse 11a, 07745 Jena, Germany, Fax: (+49) 3641-656-679
Search for more papers by this authorWe thank our colleagues at the HKI department of Biomolecular Chemistry for providing NMR and MS data. We are grateful to Dr. L. P. Martinova, Central Research Institute of Tuberculosis of the Russian Academy of Science, for biological testing. Financial support by the German Ministry for Education and Research (BMBF, CHN 02/322) is gratefully acknowledged.
Graphical Abstract
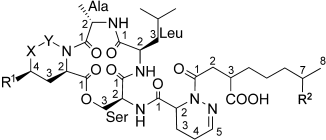
Out of the brew! Four new antibiotics were isolated from a fermentation broth of Steptomyces lydicus (strain HKI0343). The 13-membered-ring peptides (see formula, XY: CH2NH, CHNH; R1, R2: H, OH) are cyclized through a lactone function at serine and also contain the nonproteinogenic amino acid piperazic acid (or a derivative thereof). The peptides show promising activity against various mycobacteria without being cytotoxic.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2006/z503381_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1S. Grabley, I. Sattler in Modern Methods of Drug Discovery (Eds.: ), Birkhäuser, Basel, 2001, pp. 87–107.
- 2S. Grabley, R. Thiericke in Drug Discovery from Nature (Eds.: ), Springer, Berlin, 1999, pp. 124–148.
- 3I. Schmid, I. Sattler, S. Grabley, R. Thiericke, J. Biomol. Screening 1999, 4, 13–23.
- 4U. Gräfe, R. Schlegel, M. Ritzau, W. Ihn, K. Dornberger, C. Stengel, W. F. Fleck, W. Gutsche, A. Härtl, E. F. Paulus, J. Antibiot. 1995, 48, 19–25.
- 5M. Chu, R. Mierzwa, L. He, L. Xu, F. Gentile, J. Terracciano, M. Patel, L. Miesel, S. Bohanon, C. Kravec, C. Cramer, T. O. Fischman, A. Hruza, L. Ramanathan, P. Shipkova, T.-M. Chan Tetrahedron Lett. 2001, 42, 3549–3551.
- 6K. Fujii, Y. Ikai, T. Mayumi, H. Oka, M. Suzuki, K. Harada, Anal. Chem. 1997, 69, 3346–3352.
- 7D. E. Williams, M. Craig, C. F. B Holmes, R. J. Andersen, J. Nat. Prod. 1996, 59, 570–575.
- 8Product information 48895 from Pierce (Rockford, IL).
- 9CCDC GNANVOG, NUDWIS, VAFPEX, and ZEMHEE contain the complete crystallographic data for compounds cited in comparison. The data can be obtained free of charge from the Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif.
- 10The R configuration was assigned arbitrarily for C-2 of PBB2. The configuration of this stereocenter should have no significant influence on the conformational arrangement of the peptide core, in particular the PBB1—Ala unit.
- 11K. Umezawa, K. Nakazawa, Y. Ikeda, H. Naganawa, S. J. Kondo, J. Org. Chem. 1999, 64, 3034–3038.
- 12H. M. Dahse, B. Schlegel, U. Gräfe, Pharmazie 2000, 55, 489–491.
- 13F. Sarabia, S. Chammaa, A. S. Ruiz, L. M. Ortiz, F. J. Herrera, Curr. Med. Chem. 2004, 11, 1309–1332.
- 14C. E. Ballard, H. Yu, B. Wang, Curr. Med. Chem. 2002, 9, 471–498.
- 15Dictionary of Natural Products on CD-ROM, Version 14.1, Chapmann & Hall, London, 2005.
- 16I. Namatame, H. Tomoda, S. Ishibashi, S. Omura, Proc. Natl. Acad. Sci. USA 2004, 101, 737–742.
- 17J. Shoji, H. Hinoo, T. Hattori, K. Hirooka, Y. Kimura, T. Yoshida, J. Antibiot. 1989, 42, 1460–1464.
- 18Y. Hathout, Y. P. Ho, V. Ryzhov, P. Demirev, C. Fenselau, J. Nat. Prod. 2000, 63, 1492–1496.
- 19K. Mochizuki, K. Ohmori, H. Tamura, Y. Shizuri, S. Nishiyama, E. Miyoshi, S. Yamamura, Bull. Chem. Soc. Jpn. 1993, 66, 3041–3046.
- 20T. Morino, A. Masuda, M. Yamada, M. Nishimoto, T. Nishikiori, S. Saito, N. Shimada, J. Antibiot. 1994, 47, 1341–1343.
- 21K. Sugawara, S. Toda, T. Moriyama, M. Konishi, T. Oki, J. Antibiot. 1993, 46, 928–935.
- 22D. Schwartz, personal communication.
- 23L. Han, X. Huang, I. Sattler, H. M. Dahse, H. Fu, W. Lin, S. Grabley, J. Nat. Prod. 2004, 67, 1620–1623.
- 24Deutsches Arzneibuch, 9th ed., Deutscher Apotheker Verlag, Stuttgart, 1986, pp. 47–48 and 424–430.
- 25S. Afonin, R. W. Glaser, M. Berditchevskaja, P. Wadhwani, K.-H. Gührs, U. Möllmann, A. Perner, A. S. Ulrich, ChemBioChem 2003, 4, 1151–1163.
- 26L. Heinisch, S. Wittmann, T. Stoiber, A. Berg, D. Ankel-Fuchs, U. Möllmann, J. Med. Chem. 2002, 45, 3032–3040.
- 27National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, Approved Standard. NCCLS Document M7A4, 5th ed. National Committee for Clinical Laboratory Standards, Villanova, PA, 2000.