Optically Active Conjugated Polymers Prepared from Achiral Monomers by Polycondensation in a Chiral Nematic Solvent†
Hiromasa Goto Dr.
Tsukuba Research Center for Interdisciplinary Materials Science (TIMS), Institute of Materials Science, University of Tsukuba, Tsukuba, Ibaraki 305-8573, Japan, Fax: (+81) 29-855-7440
Search for more papers by this authorKazuo Akagi Prof. Dr.
Tsukuba Research Center for Interdisciplinary Materials Science (TIMS), Institute of Materials Science, University of Tsukuba, Tsukuba, Ibaraki 305-8573, Japan, Fax: (+81) 29-855-7440
Search for more papers by this authorHiromasa Goto Dr.
Tsukuba Research Center for Interdisciplinary Materials Science (TIMS), Institute of Materials Science, University of Tsukuba, Tsukuba, Ibaraki 305-8573, Japan, Fax: (+81) 29-855-7440
Search for more papers by this authorKazuo Akagi Prof. Dr.
Tsukuba Research Center for Interdisciplinary Materials Science (TIMS), Institute of Materials Science, University of Tsukuba, Tsukuba, Ibaraki 305-8573, Japan, Fax: (+81) 29-855-7440
Search for more papers by this authorThis work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Graphical Abstract
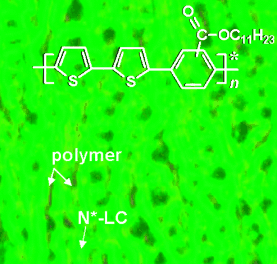
Contagious chirality: A chiral polymer (see image) was obtained from the polycondensation of an achiral monomer by using a chiral nematic liquid crystal (N*-LC) as an inert reaction solvent. The chirality of the polymer is thought to derive from the asymmetry produced by the chiral medium during polymerization.
References
- 1
- 1aN. Cramer, S. Laschat, A. Baro, H. Schwalbe, C. Richter, Angew. Chem. 2005, 117, 831–833; Angew. Chem. Int. Ed. 2005, 44, 820–822;
- 1bV. Athawale, M. Athawale, N. Manjrekar, J. Polym. Mater. 2004, 21, 305–313;
- 1cP. S. Baran, J. M. Richter, D. W. Lin, Angew. Chem. 2005, 117, 615–618;
10.1002/ange.200462048 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 609–612;
- 1dM. M. Ahmed, B. P. Berry, T. J. Hunter, D. J. Tomcik, G. A. O'Doherty, Org. Lett. 2005, 7, 745–748;
- 1eC. Naeslund, S. Ghirmai, S. Sjoeberg, Tetrahedron 2005, 61, 1181–1186;
- 1fK. Tanaka, G. Nishida, A. Wada, K. Noguchi, Angew. Chem. 2004, 116, 6672–6674; Angew. Chem. Int. Ed. 2004, 43, 6510–6512;
- 1gA. J. Boydell, V. Vinader, B. Linclau, Angew. Chem. 2004, 116, 5795–5797; Angew. Chem. Int. Ed. 2004, 43, 5677–5679;
- 1hM. Rivero, I. Alonso, J. C. Carretero, Chem. Eur. J. 2004, 10, 5443–5459;
- 1iD. G. Gillingham, O. Kataoka, S. B. Garber, A. H. Hoveyda, H. Amir, J. Am. Chem. Soc. 2004, 126, 12 288–12 290;
- 1jK. Fuji, T. Morimoto, K. Tsutsumi, K. Kakiuchi, Kiyomi, Tetrahedron Lett. 2004, 45, 9163–9166;
- 1kA. Cordova, Chem. Eur. J. 2004, 10, 1987–1997;
- 1lA. H. Hoveyda, Stimul. Concepts Chem. 2000, 145–160.
- 2
- 2aH. Wynberg, J. Macromol. Sci. A 1989, 26, 1033–1041;
- 2bK. Soai, I. Sato, Organomet. News 2003, 4, 138–141;
- 2cT. Shibata, T. Hayase, J. Yamamoto, K. Soai, Tetrahedron: Asymmetry 1997, 8, 1717–1719.
- 3
- 3aL. Shengjian, J. Yaozhong, M. Aiqiao, Y. Guishu, J. Chem. Soc. Perkin Trans. 1 1993, 885–886;
10.1039/p19930000885 Google Scholar
- 3bA. H. Alberts, H. Wynberg, J. Am. Chem. Soc. 1989, 111, 7265–7266;
- 3cK. Soai, Y. Inoue, T. Takahashi, T, Shibata, Tetrahedron 1996, 52, 13 355–13 362.
- 4
- 4aC. Cimarelli, A. Mazzanti, G. Palmieri, E. Volpini, J. Org. Chem. 2001, 66, 4759–4765;
- 4bB. M. Matute, J.-E. Baeckvall, J. Org. Chem. 2004, 69, 9191–9195;
- 4cS. Miniere, V. Reboul, P. Metzner, M. Fochi, B.-F. Bonini, Tetrahedron: Asymmetry 2004, 15, 3275–3280;
- 4dO. Hamelin, J. Pecaut, M. Fontecave, Chem. Eur. J. 2004, 10, 2548–2554;
- 4eP. Jakubec, D. Berkes, F. Povazanec, Tetrahedron Lett. 2004, 45, 4755–4758;
- 4fM. Edin, J. Steinreiber, J.-E. Baeckvall, Proc. Natl. Acad. Sci. USA 2004, 101, 5761–5766.
- 5
- 5aC. M. Chung, M. Hasegawa, J. Am. Chem. Soc. 1991, 113, 7311–7316;
- 5bR. Bielski, M. Tencer, Can. J. Chem. 2003, 81, 1029–1037;
- 5cL. Addadi, J. van Mill, M. Lahav, J. Am. Chem. Soc. 1982, 104, 3422–3429.
- 6Y. Okamoto, T. Nakano, Chem. Rev. 1994, 94, 349–372.
- 7
- 7aS. Yorozuya, I. Osaka, A. Nakamura, Y. Inoue, K. Akagi, Synth. Met. 2003, 135–136, 93–94;
- 7bB. M. W. Langeveld-Voss, R. A. J. Janssen, E. W. Meijer, J. Mol. Struct. (THEOCHEM) 2000, 521, 285–301;
- 7cK. Giseop, T. Masuda, J. Polym. Sci. Part A 2001, 39, 71–77;
- 7dH. Goto, K. Akagi, Synth. Met. 2001, 119, 165–166;
- 7eH. Tang, M. Fujiki, T. Sato, Macromolecules 2002, 35, 6439–6445.
- 8
- 8aS. Su, N. Kuramoto, Macromolecules 2001, 34, 7249–7256;
- 8bE. Yashima, Y. Maeda, Y. Okamoto. Chem. Lett. 1996, 955–956.
- 9
- 9aK. Akagi, G. Piao, S. Kaneko, K. Sakamaki, H. Shirakawa, M. Kyotani, Science 1998, 282, 1683–1686;
- 9bH. Shirakawa, Curr. Appl. Phys. 2001, 1, 88–89;
- 9cG. Piao, N. Kawamura, K. Akagi, H. Shirakawa, M. Kyotani, Polym. Adv. Technol. 2000, 11, 826–829.
- 10
- 10aH. Goto, K. Akagi, Macromolecules 2005, 38, 1091–1098;
- 10bH. Goto, K. Akagi, Macromol. Rapid Commun. 2004, 25, 1482–1486;
- 10cH. Goto, K. Akagi, Jpn. Pat. 362 979, 2002 [ Chem. Abstr. 2003, 139, 344 169];
- 10dH. Goto, K. Akagi, Book Abstr. Int. Conf. Sci. Technol. Synth. Met. (ICSM) 2002 (Shanghai, China) 2002, p. 17.
- 11
- 11aN. Berova, K. Nakanishi in Circular Dichroism: Principles and Applications, 2nd ed. ), Wiley-VCH, New York, 2000, pp. 337–382;
- 11bS. F. Mason, Molecular Optical Activity and the Chiral Discriminations, Cambridge University Press, Cambridge 1982.
- 12
- 12aL. Groenendaal, M. J. Bruining, E. H. J. Hendrickx, A. Persoons, J. A. J. M. Vekemans, E. E. Havinga, E. W. Meijer, Chem. Mater. 1998, 10, 226–234;
- 12bO. Henze, D. Parker, W. J. Feast, J. Mater. Chem. 2003, 13, 1269–1273.
- 13M. M. Green, N. C. Peterson, T. Sato, A. Teramoto, R. Cook, S. Lifson, Science 1995, 268, 1860–1866.
- 14
- 14aL J. Prins; P. Timmerman, D. N. Reinhoudt, J. Am. Chem. Soc. 2001, 123, 10 153–10 163;
- 14bB. M. W. Langeveld-Voss, R. J. M. Waterval, R. A. J. Janssen, E. W. Meijer, Macromolecules 1999, 32, 227–230;
- 14cM. M. Green, M. P. Reidy, R. J. Johnson, G, Darling, D. J. O'Leary, G. Willson, J. Am. Chem. Soc. 1989, 111, 6452–6454;
- 14dA. Saxena, G. Guo, M. Fujiki, Y. Yang, A. Ohira, K. Okoshi, M. Naito, Macromolecules 2004, 37, 3081–3083;
- 14eN. Reinhoudt, M. C. Calama, Science 2002, 295, 2403–2406.